What is Surfactants?
Surfactants mean surface-active agents and they play a significant part in most cleaning products. To clean the dirt on surfaces, the water needs to be able to reach the surface. With surfactants, water can get to the surface as it reduces the water tension and separates the water from the impurities hence it traps impurities which makes it to be very effective in cleaning.
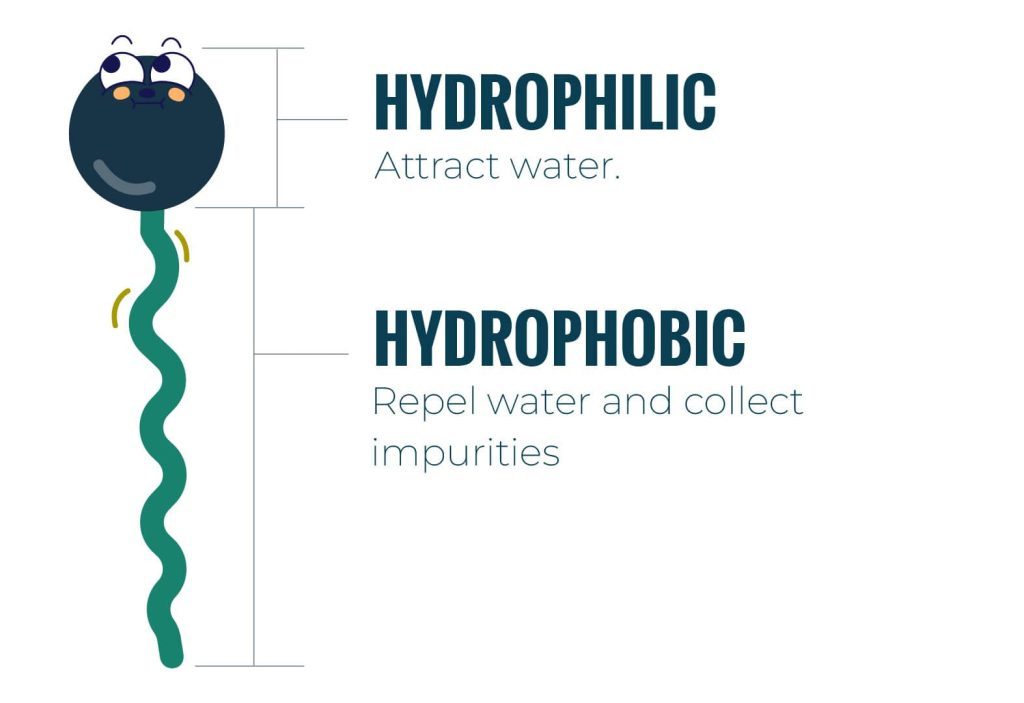
The 2 Ends of a Surfactant
Every surfactant has two ends. One end wants to be in the water and the other does not. The top part which looks like the head is called Hydrophilic which doesn’t like to be in the water and prefer surrounded by soil while the tail consists of is a hydrocarbon, fluorocarbon, or siloxane which want to in the water.
How does it work?
What this does is it helps to attract impurities (similarly to a magnet) and surround them, while the hydrophilic heads pull the surrounded soils off the surface and into the cleaning solution. Then the micelles reform with the tails gathering the impurities in the center of the structure. It is also important to know that some surfactants are very good at removing some types of impurities but not very good at removing others.
Structure of Surfactant Molecules
The surfactant structure includes a head and a tail, both of which have opposing properties that make the molecule an ideal cleaning agent. The head has charged atoms. Due to this, it dissolves in water. Or in other words, it’s hydrophilic. The tail, on the other hand, does not have any charged atoms, so it is hydrophobic.
Surface Tension
The water molecules (H₂O) are attracted to each other. The slight positive charges on the hydrogen atoms in a water molecule attract the slight negative charges on the oxygen atoms of other water molecules. The molecules deep inside the bulk are attracted equally in all directions. However, the molecules at the surface experience no upward pull from other molecules, so these molecules only experience a downward pull from molecules beneath the surface.
@orapi_recommends Ever wondered how soaps and detergents magically remove all dirt and grime? 🤔 If you answer SURFACTANTS, you're right! ✅ Surfactants are surface-active agents, and they play a significant part in most cleaning products.🧼 Surfactants reduce water tension and separate the water from the impurities. 💧 Finally, they trap the impurities which makes cleaning quick and easy! 🏃🏼♂️ This video provides a visual description of the entire process! 📹 #CleaningTips #cleantok #CleaningChemistry #Surfactants #Micelles ♬ original sound - ORAPI Recommends
Mechanism of Surfactant
Now, when the surfactant molecule enters the water, the hydrophilic head wants to stay dissolved, but the hydrophobic tail wants to stay out of the water. Therefore, the head stays submerged in water, whereas the tail stays out of it. Because the head has positive and negative charges, it is attracted by other molecules, but the tail is repelled by water. These forces cancel out each other.
You might be asking, “so, how does it reduce the tension on the surface?” Firstly, as the number of surfactant molecules increases, it occupies more area on the surface. The density of the water molecules decreases—the lesser the water molecules on the surface, the lesser the tension.
The second thing to consider is that the head attracts the water molecules on the surface, which reduces the downward pull of the experience from the molecules underneath. The tail prevents the surfactant molecule itself from going deep. This reduces the surface tension, and the tail prevails in gathering impurities right from the center of the bulk.
Types of Surfactants
The hydrophilic head of the surfactant can have a positive, negative, or neutral charge. Accordingly, it may be classified as anionic, nonionic, cationic, or amphoteric.
Anionic Surfactant
The head ends of these surfactants have a negative charge that helps the surfactant molecules lift and suspend soils in micelles. They are frequently used in soaps and detergents because they target a wide range of soils. Anionic surfactants also create a lot of foam when mixed.
Gluconates, sulfonates, and sulfates are some examples of anionic surfactants.
Nonionic Surfactants
Nonionic surfactants are neutral, lacking any charge on their hydrophilic ends. These are excellent at emulsifying soils and are better than anionic surfactants at removing organic soils. Both surfactants are often mixed to create a hybrid, dual-action cleaner that can lift and particulate soils as well as emulsify oily soils.
Nonionic surfactants can be both non-foaming and low-foaming, making them ideal for low-foaming detergents.
Cloud point, a feature unique to nonionic surfactants, is the temperature at which the nonionic surfactant begins to separate from the cleaning solution. At this point, the solution becomes cloudy. For low-foaming cleaners, the optimal detergency is at the cloud point, and for foaming cleaners, the optimal detergency is either at the start of the cloud point or just below it.
The cloud point temperature depends on the proportion of hydrophobic and hydrophilic ends in the solution. Some nonionic surfactants reach the cloud point at room temperature, while others don’t have a cloud point because the ratio of hydrophilic to hydrophobic moieties is higher.
Examples of nonionic surfactants include alkoxylates, ethoxylates, and cocamide
Cationic Surfactants
Cationic surfactant molecules have a positive charge on their ends. The positive charge makes them ideal for use on anti-static products like fabric softeners. In addition, because of their antimicrobial properties, they are also used as disinfectants.
Cationic and anionic surfactants are incompatible because of their opposing charges (positive and negative, respectively). However, cationic surfactants and nonionic surfactants work well together.
Alkyl ammonium chloride is a good example of a cationic surfactant.
Amphoteric Surfactant
Amphoteric surfactants have dual charges on the head ends of the structure. The positive and the negative charges cancel each other out and create a net of zero called the zwitterionic. How the surfactant acts depends on the pH of the solution. In acidic solutions, the surfactant will take the properties of cationic surfactants, and in alkaline solutions, it will behave similarly to anionic surfactants.
They are frequently used in personal care products such as cosmetics and shampoos. Examples include amino oxides, betaines, and amphoteric surfactants.
Surfactant in Cleaner
Surfactants are a key ingredient in cleaning products. They are often mixed with solvents and inhibitors to strengthen their effectiveness in removing grease, grime, and fuel oils.
Moreover, biodegradable surfactants reduce the environmental impact of the products by breaking down at a much faster pace than their regular counterparts.
ORAPI offers a wide range of high-quality biodegradable surfactant cleaners for various needs. Read the technical datasheet for each product or send us an inquiry to know more.