Biofilms are a grave global nuisance due to their ability to make themselves immune to antibiotics and external stresses and resistant to antimicrobial agents; therefore, it contributes to persistent chronic infections and damage.
Moreover, they are of concern to the food and beverage processing and manufacturing industries because a biofilm is not only difficult to remove from food processing and manufacturing equipment but also equally challenging to identify. Therefore, causing a variety of issues ranging from energy wastage and equipment spoilage to retaining harmful pathogenic bacteria such as Listeria. In addition, the presence of water and nutrient sources facilitates the development of biofilms. As such, this problem persists in our industry.
This article will give you a better understanding of the features of biofilms and the measures one must take to identify, remove, and keep them under control.
What is a Biofilm?
Biofilms are communities of microorganisms, mainly bacteria, contained in extracellular polymers or exopolysaccharide matrices.
“Exopolysaccharides (EPS) are extracellular macromolecules excreted as tightly bound capsule or loosely attached slime layer in microorganisms” (Angelin & Kavitha, 2020).

They can grow on different surfaces, such as the main supply and the internal walls of drinking water pipes, food-contact surfaces, recycled water storage areas, and cooling towers. Thus, they can be a continuous source of pathogens and cause damage, such as metal pipe corrosion
Owing to its structure, biofilm protects the microorganisms inside from external attacks and disinfectants. As a result, a wide variety of bacteria exchange genetic material and phenotypic characteristics to form vast biofilm layers. This occurs through plasmid exchange, which refers to the transfer of substances such as nutrients, gases, and waste products between cells and their surrounding environment,—this can occur through various means, including diffusion, osmosis, and active transport—allowing them to obtain the substances they need and get rid of waste products.
Risks Associated With Biofilms
The existence of biofilms poses several risks in the food and beverage industry. We can separate these risks into two categories: food safety and operating effectiveness.
Food Safety
- Changes in sensory properties (also called organoleptic alterations) of substance such as appearance, smell, taste or texture.
- Initiating changes in other properties of the product, such as its shelf-life.
- Cross-contamination, potentially also with pathogenic bacteria.
Operating Effectiveness
- Blockage in pipes, opening holes, filters, and valves.
- Contamination in sampling and measurement systems.
- Corrosion.
- Changes in surface properties.
- Reduction in heat transfer ratio.
- Inhibition of the biocidal activity
Listeria spp.
Listeria is a genus of bacteria that includes several species, including Listeria monocytogenes, which is the most well-known and is the cause of a serious infection known as listeriosis. Listeria bacteria are widely distributed in the environment and can be found in soil, water, and animal faeces. They can also contaminate food, particularly raw or uncooked animal products such as meat, poultry, and dairy products. Listeriosis is a rare but potentially life-threatening infection that can cause fever, muscle aches, gastrointestinal symptoms, and more severe complications such as meningitis or sepsis.
Biofilm provides sufficient protection to Listeria spp. Therefore, it is commonly linked to biofilm in the food and beverage industry.
Listeria spp. has a low capacity to form biofilms in and on itself. So, it is often found in multi-species biofilms, where other bacteria—such as lactobacillus, Plantarum, and flavobacteria—are also present. However, upon exposure to low temperatures (lower than 0°C), Listeria spp. goes into self-protection mode, and its capacity to form biofilms increases.
Listeria spp. has a low capacity to form biofilms in and on itself. So, it is often found in multi-species biofilms, where other bacteria—such as lactobacillus, Plantarum, and flavobacteria—are also present. However, upon exposure to low temperatures (lower than 0°C), Listeria spp. goes into self-protection mode, and its capacity to form biofilms increases.
It is worth noting that Listeria spp. grows more slowly in biofilm than in columns of water (free planktonic form), which allows it to adhere to all sorts of materials. Listeria monocytogenes have been found on cutting boards, cheese seasoning scales, trolleys, storage tanks, conveyor belts, production hall floors, and throughout processing and packaging plants.
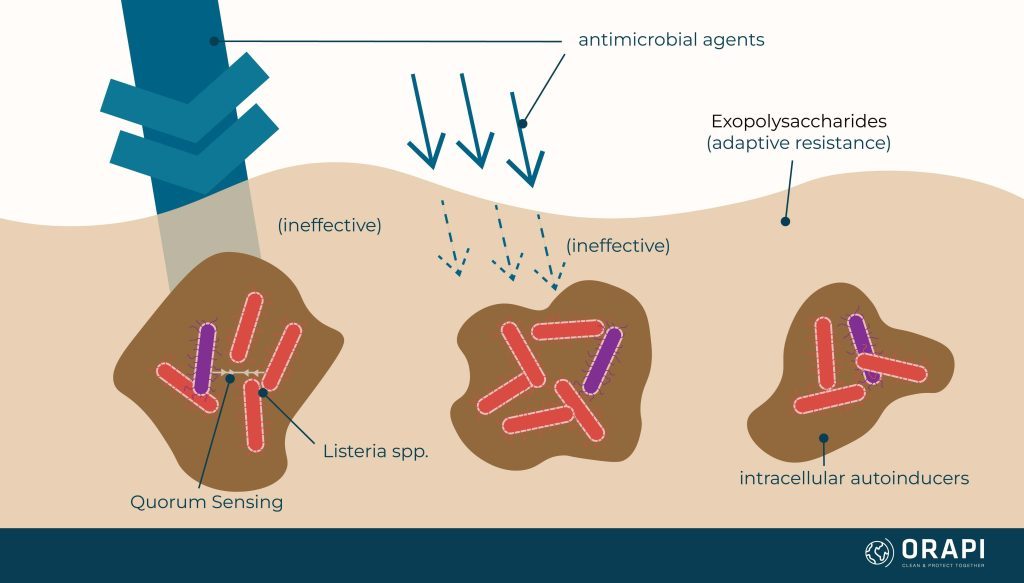
Biofilm's Adaptive Resistance
Biofilm communities can cause physiological and metabolic changes in the host cells. This can promote particle chemical neutralisation of active ingredients. For example, an oxidiser can be rendered less effective by the EPS (or the biofilm) before it reaches the target cells. In addition, a concentration gradient occurs at the biofilm layer, reducing disinfectant activity and effectiveness.
Quorum Sensing
Quorum sensing is a process by which bacteria communicate and coordinate their behaviour by releasing and detecting chemical signalling molecules called autoinducers. This process allows bacteria to sense the presence and density of other bacteria in their environment and to coordinate their gene expression and behaviour in response. Quorum sensing is thought to play a role in a variety of bacterial behaviours, including the formation of biofilms, the production of virulence factors, and the regulation of gene expression. Understanding quorum sensing and how it works could lead to the development of new approaches for controlling or preventing bacterial infections.

Scientific evidence suggests that one species can influence quorum sensing to influence the biofilm formation of other species. As a result, the species present within the biofilm can organise themselves to utilise the nutrients more efficiently, resist adverse environmental conditions, and even build a more robust EPS for species lacking the same ability.
It is important to remember that microbial species cooperate to acquire greater resistance, also known as adaptive resistance, to antimicrobial agents through specific strategies such as:
- The extracellular polymeric matrix has multiple compositions: the extracellular polymeric matrix (EPM) is a term used to describe the material surrounding bacterial cells within a biofilm.
- Harbouring anaerobic bacteria: the aerobic species consume oxygen, creating an anaerobic environment inside the EPS, allowing anaerobic bacteria to grow inside.
- One species can induce changes in another, making it more resistant to antimicrobial agents.
Greater gene expression that increases resistance is especially evident in microorganisms attached to the surface. Two examples of such microorganisms are:
Acid Shock Proteins (ASP) repair cellular damage from acid stress.
Acid shock proteins (ASPs) are a class of proteins that are expressed in response to acidic conditions. They are involved in a variety of functions, including the maintenance of pH homeostasis, the repair of damaged proteins, and the regulation of gene expression. ASPs are typically expressed at high levels in bacteria when exposed to acidic conditions, such as those that can occur in the stomach or during fermentation. The expression of ASPs is thought to help bacteria survive and adapt to these stressful conditions. Some ASPs have also been shown to have antimicrobial activity, suggesting that they may play a role in defence of bacteria against other microorganisms.
Heat Shock Proteins (HSP) repair cellular damage from thermal stress.
Heat shock proteins (HSPs) are a class of proteins produced in response to various types of stress, including heat stress, oxidative stress, and other cellular stress. They play a role in the protection and repair of damaged proteins, as well as in the maintenance of protein folding and stability. HSPs are found in all organisms, from bacteria to humans, and are classified based on size and function. HSPs are typically produced at high levels in response to stress, and their expression is regulated by a group of transcription factors known as heat shock factors. HSPs are thought to play a role in the ability of cells to survive and adapt to stress, and they have been studied for their potential use in the treatment of various diseases.
How Biofilms Form
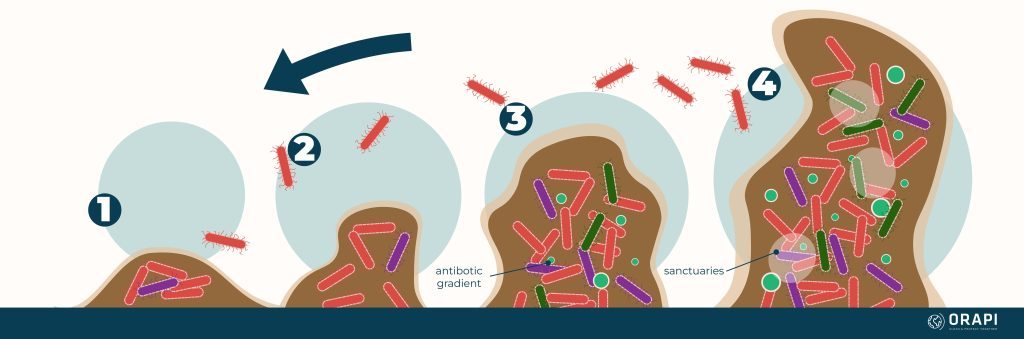
1. Attachment
2.Growth & Proliferation
3.Maturation
4.Dispersal
The biofilm may eventually break down, and the microorganisms may be released back into the environment, forming new biofilms or entering a planktonic (free-floating) phase.
It is important to note that the biofilm formation process can vary depending on the species of microorganisms involved, the environmental conditions, and other factors.
Controlling, Identifying, and Removing Biofilms
Identifying Biofilm
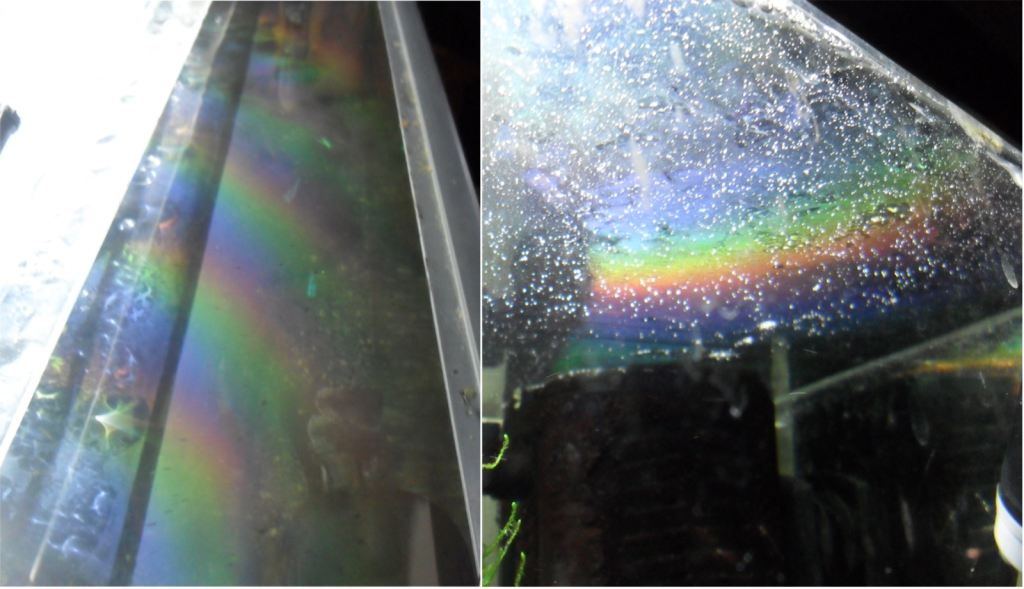
Two common methods of determining the presence of biofilms are:
- Visual: You can detect a ‘rainbow’ appearance on stainless steel or an abnormal film covering equipment
- Tactile: Upon touching a seemingly clean equipment, if you notice a slimy feeling, it’s a clear-cut sign of biofilm growth.
More complicated methods include:
- Microscopy: Microscopy techniques, such as brightfield microscopy, fluorescence microscopy, or confocal laser scanning microscopy, can be used to visualise biofilms and examine their structure.
- Culture-based methods: Biofilms can be grown in the laboratory and then cultured on solid media or in liquid media to assess their growth and biomass.
- Spectrophotometric methods: The amount of biofilm biomass can be measured using spectrophotometric methods, which involve the use of light absorbancy to quantify the amount of biomass present.
- Fluorescent staining: Biofilms can be stained with fluorescent dyes or probes that specifically bind to biofilm components, such as extracellular polymeric substances (EPS), and then visualised using microscopy or other imaging techniques. However, it is important to note that many biofilm detection tools using colouring methods such as safranin, eosin, methylene blue, or crystal violets typically highlight organic residue (which may or may not contain biofilm).
- Molecular methods: Biofilms can also be detected and characterised using molecular techniques, such as polymerase chain reaction (PCR)—polymerase chain reaction (PCR) is a laboratory technique that is used to amplify or replicate small amounts of DNA—which you can use to amplify and detect specific genetic markers associated with biofilm formation.
Controlling
Hygienic Design
Equipment should be not only accessible for sanitation but also inspectable, with regular inspections scheduled at frequent intervals.
It would be best if you tried to exclude blind spots and shaded areas from the design as much as possible and implement hygienic design following the industry standards across all production equipment processes. These include but are not limited to the following:
- ISO 14159:2013: This standard covers the hygienic design of equipment used in the food industry, including guidelines for materials, surfaces, and shapes that minimise the risk of contamination.
- ISO 22000:2018: This standard covers food safety management systems, including guidelines for hygienic design in the construction and maintenance of food processing and handling facilities.
Avoid activities leading to corrosion, pitting of food contact surfaces, and abrasion at all costs, as cracks and holes in poorly welded surfaces present risks of microbial contamination. The OEM’s (original equipment manufacturer) instructions should be referred to when changing the gaskets and seals.

Easily wearable materials can become rough and porous. Therefore, you should replace them frequently before they become a source of contamination. The replacement frequency should be guided by the Master Sanitation Schedule, i.e., the document that outlines the specific steps that need to be taken to clean and disinfect a facility or equipment on a regular basis.
Cleaning, Disinfection, and Sanitisation
Some vital basic principles that you should take into consideration are:
- Refer to the SSOPs (Sanitation Standard Operating Procedures): Sanitation Standard Operating Procedures (SSOPs) are a set of written instructions that outline the specific steps that need to be followed to clean and disinfect a facility or equipment. They are designed to ensure that a facility or equipment is consistently and effectively cleaned and disinfected in order to minimise the risk of contamination or the spread of illness.
- Every phase of the cleaning process should be followed.
- Adhere to the specific values or verified working parameters to ensure that the system, process, product, or equipment is operating correctly and consistently. These parameters may include variables such as temperature, pressure, humidity, concentration, turbulent flow, mechanical action, action time, rate, or other factors relevant to the specific systems or process.
- Check to ensure that the cleaning or sanitisation/disinfection solution reaches all areas of the equipment (ensure all surface areas are covered, look for blind spots in CIP (cleaning in the process) and OPC cleaning, etc.).
- Disassemble the equipment to reach inaccessible parts.
- Avoid insufficient cleaning and make sure that no films or deposits are left behind after cleaning.
- Check for aerosols during cleaning. Aerosols can carry bacteria and cause cross-contamination.
- As a part of the post-cleaning ritual, perform a visual and analytical check of the equipment and all critical surfaces.
Never underestimate the value of a good scrub during manual cleaning. Mechanical action is one of the four key elements of achieving effective cleaning (together with the contact time, temperature, and chemical action). The perfect balance of these factors forms the most effective Sinner’s Circle.
Monitor and Detect Biofilm
A comprehensive approach which includes a range of procedures and apparatus and a protocol defined by specific requirements should be used. Some of these are listed below:
- ATP (bioluminescence) and rapid detection of specific microorganisms through specific tests: Adenosine triphosphate (ATP) bioluminescence is a method used to rapidly detect the presence of microorganisms. ATP is a molecule that is present in all living cells and is involved in energy metabolism. The bioluminescence assay is based on the fact that when ATP is broken down, it releases energy that can be measured as light.
- Semi-quantitative detection of proteins or sugars: it refers to the use of analytical techniques to determine the relative amount of proteins or sugars present in a sample. These techniques cannot precisely measure the number of proteins or sugars present, but they can give an estimate of the relative concentration of these molecules.

- Borescope or endoscope for pipe and tank inspection: A borescope, also known as an endoscope, is a specialised device that is used to inspect the inside of pipes, tanks, and other types of enclosures. It consists of a flexible tube with a camera and light at one end, inserted into the enclosure through a small opening. The camera and light transmit images of the inside of the chamber to a viewing device, allowing the user to see the interior in real time.
- Monitoring parameters in CIP programs: In a CIP (clean-in-place) program, it is essential to monitor the mechanical and hydraulic parameters of the equipment and systems being cleaned to ensure that the cleaning process is effective and consistent. You can do this through flow metres and/or digital platforms.
- Shadow tests on open surfaces after cleaning: To perform a shadow test, a sample of the surface being tested is collected and placed on a microscope slide. The slide is then incubated with a staining solution specific to EPS. If a biofilm is present on the surface, it will be stained by the staining solution and visible under a microscope.
- Verification of sanitation parameters (Time, Action, Chemical, Temperature) and systems such as spray balls, including their design, installation, etc., and the foaming systems: Verification of sanitation parameters is essential to address any issues that may be contributing to the persistence of biofilms. To verify that sanitation parameters are sufficient to detect biofilms, it may be necessary to test the surface after cleaning to determine if a biofilm is present with the abovementioned methods.
Removing Biofilm
Eliminating Biofilm From CIP Systems

The cleaning solution and the appropriate velocity play significant roles in effectively removing the contamination from the pipe’s inner surface and determining the success of CIP systems.
During the CIP, the flow rate of the cleaning solution will vary along the diameter of the piper: higher in the centre and lower next to the wall due to friction. This is known as ‘speed profile’.
The liquid layer on the tube surface is called the ‘sub-laminar layer’, and its speed is always equal to 0. It tends to become thinner as the fluid velocity increases, and the mechanical effect on it becomes much stronger. This is an essential procedure for removing and preventing the formation of a biofilm on piping structures in the food and beverage industry. Depending on the diameter of the tubes, a minimum thickness of the sub-laminar is attained when the fluid speed is between 1.5m/s to 2m/s.
Eliminating Biofilms From Open Surfaces
Physical, chemical or a combination of these two methods are often used to eliminate and inactivate biofilms on open surfaces.
Applying heat to the surface can help to kill the microorganisms in biofilms and make it easier to remove them. This can be done using methods such as steam cleaning or hot water cleaning. It is important to note that the water has to be between 80-90°C for effective removal. Scrubbing or scraping the surface with a brush or other tool can help to physically remove biofilms from the surface. This may be more effective when combined with a biocide and chemical removal strategy.
A chlorine detergent is an excellent choice for removing biofilms, as it can effectively penetrate the EPS matrix and kill all the microorganisms within it.
ORAPI RECOMMENDS:
Self Foaming Chlorinated Detergent
HI CHLOR is a blend of self-foaming surfactants, scale inhibitors, chlorine and fat emulsifiers to produce a safe and easy-to-use detergent combining excellent cleaning results and ease of operation.
HI CHLOR is safe on all stainless and steel surfaces when used at the recommended concentrations.
Conclusion
All in all, biofilms are composed of microorganisms embedded in extracellular polymeric substance (EPS) matrixes, which provide a physical barrier to the penetration of many antimicrobial agents. Therefore, different species of bacteria often take each other’s properties and acquire greater resistance through quorum sensing. This makes it necessary to identify, control, and remove biofilms by combining different methods before they cause any harm to health or equipment.