Cleaning products containing Quaternary Ammonium Compounds (QACs) has become increasingly prevalent, particularly in healthcare facilities, food processing, food service establishments, daycare facilities, and schools. QACs are highly effective in eliminating many pathogens, making them a popular choice for disinfection and cleaning.
However, there has been growing concern about these compounds’ potential health impacts. This article explores the use of QACs in cleaning and disinfection, their effectiveness in eradicating pathogens, their potential risks, and their far-exceeding advantages.
What Are Quaternary Ammonium Compounds?
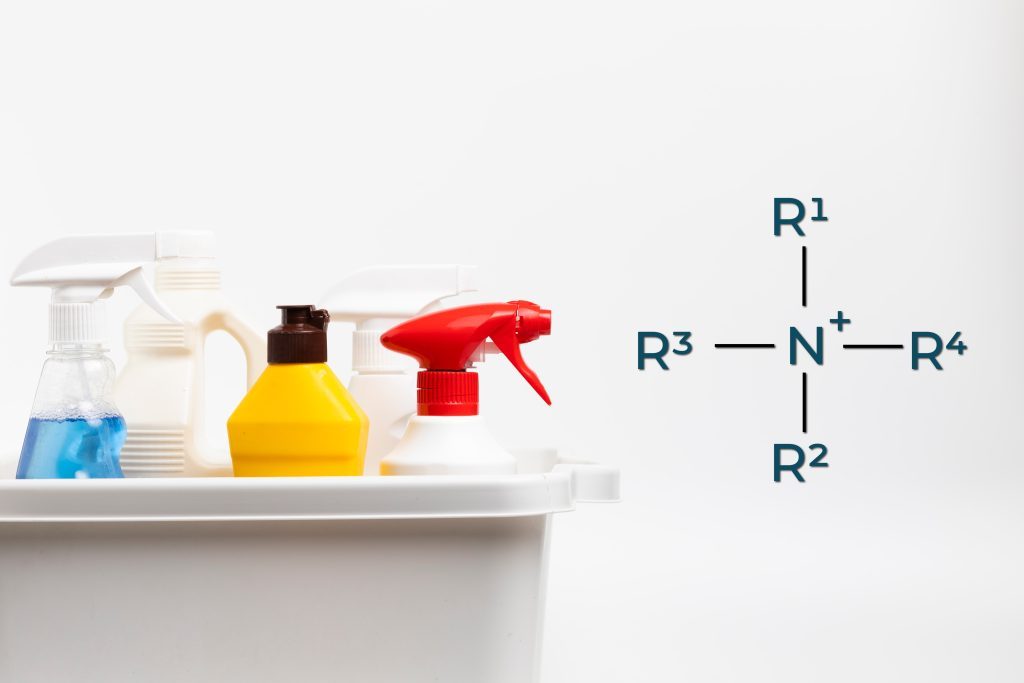
QACs, short for Quaternary Ammonium Compounds, are a class of chemicals that possess the ability to eradicate mould, viruses, fungi, and bacteria.
Commonly Used QACs

Disinfectants and sanitisers that contain QACs are available in various formulations, including liquids and foams. In addition, these compounds are frequently combined with other active ingredients, such as alcohols or hydrogen peroxide, to enhance their efficacy against specific types of microorganisms. Benzalkonium chloride, dodecylbenzene sulfonic acid, and cetyltrimethylammonium chloride are among the commonly used QACs in disinfectant formulations.
Quats
QAC is commonly called “quats” because it is an abbreviation for “quaternary ammonium compounds”, which is the full name of this class of chemicals. “Quaternary” refers to the four organic groups attached to the positively charged nitrogen atom, while “ammonium” refers to the chemical structure that includes a nitrogen atom and four hydrogen atoms. The term “compounds” simply refers to the fact that these chemicals are made up of two or more elements chemically combined.
How Does QAC Work?
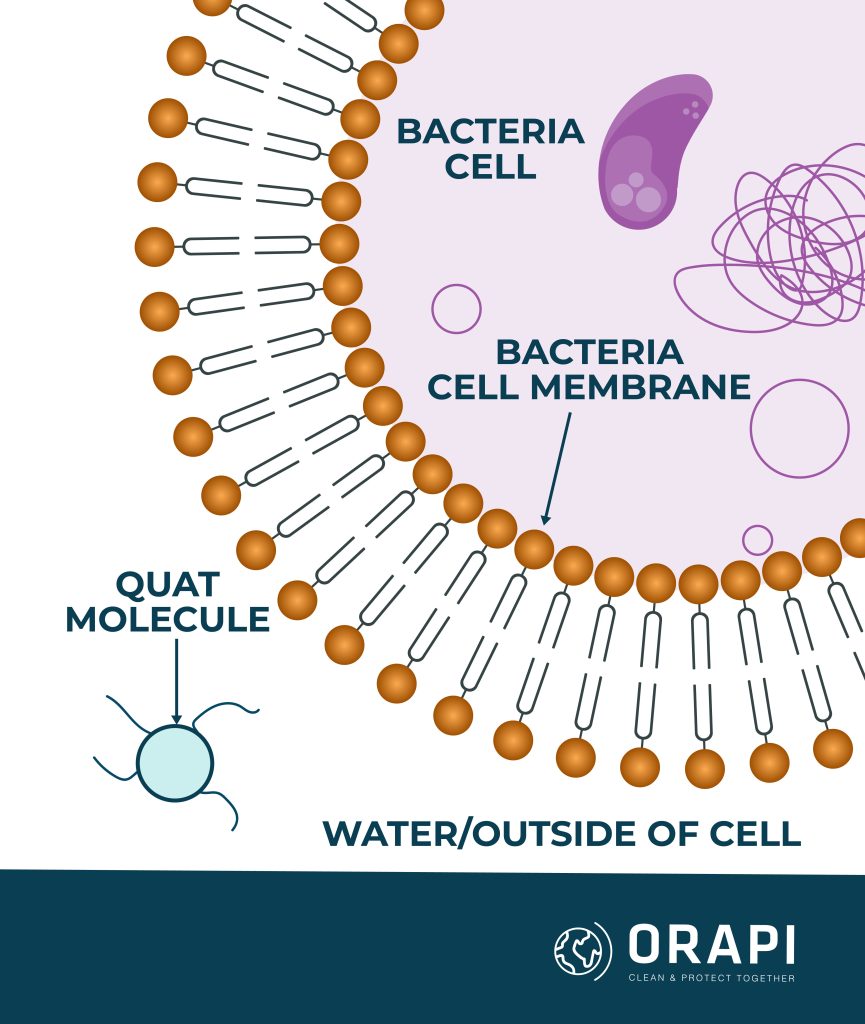
QACs are organic compounds renowned for their antimicrobial properties. These compounds possess a permanent positively charged nitrogen, facilitating their binding to negatively charged surfaces, such as cell membranes. The mechanism behind the microbicidal activity of QACs involves their adsorption (sticking) onto certain parts of the microorganisms’ cell membranes, specifically proteins or acidic phospholipids within the membrane. This adsorption leads to the formation of hydrophilic voids (gaps that are attracted to water), which ultimately cause the cell membrane to become disrupted or damaged. As a result of this damage, the microorganism is killed, hence the term “microbicidal activity”.
Properties of Quaternary Ammonium Compounds
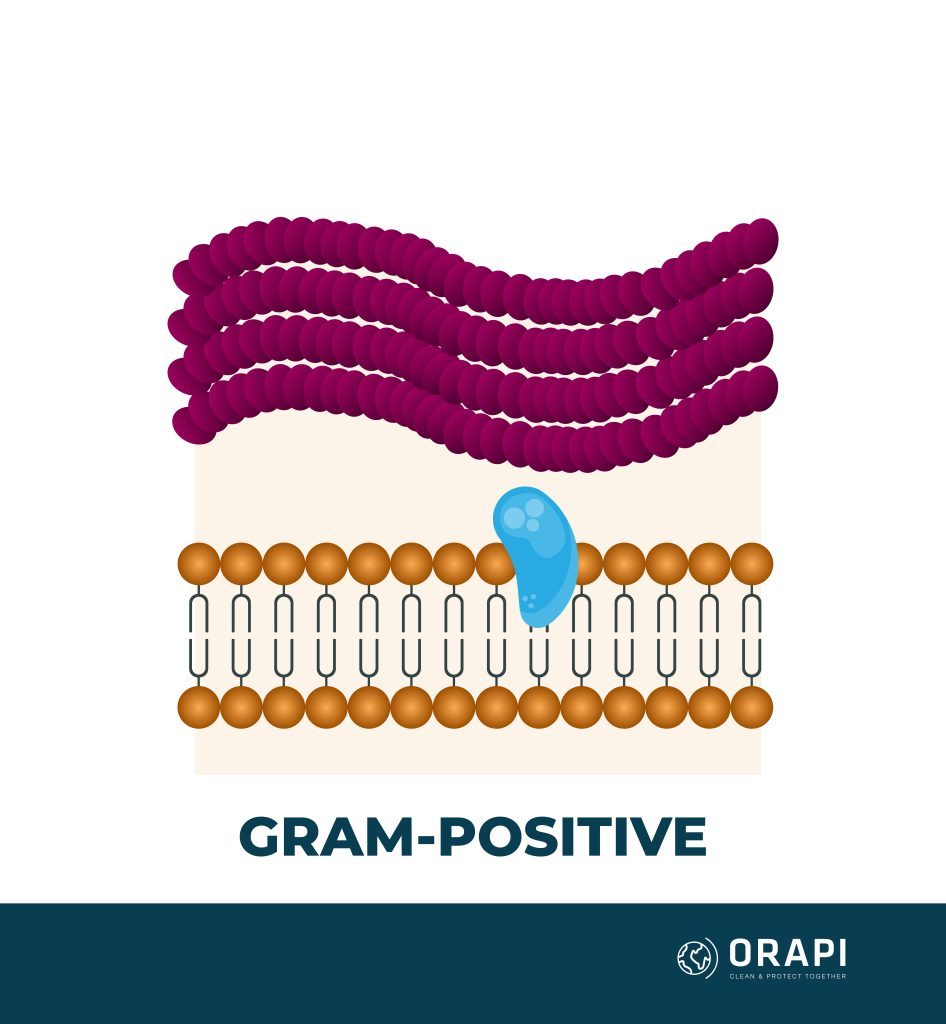
Quaternary ammonium compounds (QACs) are generally more effective against lipophilic (fat-loving) microorganisms such as Gram-positive bacteria and enveloped viruses. On the other hand, they may have limited efficacy against hydrophilic (water-loving) microorganisms such as Gram-negative bacteria and non-enveloped viruses. However, the effectiveness of QACs can vary depending on the specific compound, formulation, and surface being treated. QACs are generally more effective against a broader range of microorganisms when combined with other disinfectants.
Advantages of Quaternary Ammonium Compounds

“Quats” have broad-spectrum activity against diverse microorganisms and offer several advantages over bleach. They are odourless, colourless, and non-corrosive, making them safer for prolonged use with metal equipment and surfaces. While their antimicrobial activity is selective, they are generally as potent as bleach or chlorine solutions.
ORAPI RECOMMENDS:
Relationship Between pH and QAC
QACs are disinfectants most effective in a slightly acidic to neutral pH range, typically between 6 and 8. In this pH range, Quaternary Ammonium Compounds can disrupt the cell membranes of microorganisms, resulting in their inactivation or death.
However, if the pH of the solution is too low or too high, the effectiveness of QACs as disinfectants can be reduced. At low pH levels, the positively charged nitrogen atom in QACs can become protonated, which diminishes their positive charge and ability to interact with negatively charged cell membranes. At high pH levels, QACs can become deprotonated, which also reduces their positive charge and overall effectiveness as disinfectants.

Therefore, using QAC-based disinfectants within the recommended pH range is important to ensure their maximum effectiveness against microorganisms.
Safety Considerations for QAC Disinfectants

As previously stated, there exist numerous types of QAC elements, and certain chemicals may adversely affect your health. Nevertheless, these risks can be mitigated by following safety guidelines and wearing the appropriate protective equipment recommended for the products.
Of the different types of Quaternary Ammonium Compounds, benzalkonium chlorides and didecyl dimethyl ammonium chloride are the two most commonly associated with triggering asthma symptoms. Many forms of benzalkonium chlorides have long names that end in “ammonium chloride.” Medical studies suggest that QAC disinfectants, other than these two types, are unlikely to cause asthma if used as directed on the container.
QAC Considerations
- If light disinfection is required, cleaning solutions incorporating hydrogen peroxide can offer a more potent and safer option than those containing QACs. However, cleaning products that contain citric acid, lactic acid, or alcohol may not provide sufficient disinfection and may not serve as suitable replacements for QACs.
- Avoid using QAC spray products for cleaning, as they can cause respiratory irritation and lead to the formation of harmful disinfectant byproducts if they interact with certain indoor chemicals. Instead, apply the cleaning solution directly onto a cloth or use a disinfecting wipe. Use the stream mode on adjustable spray bottles if spraying is necessary.

- Adhering to the label instructions for dilution is crucial.
- In areas with hard water conditions, it may be necessary to increase the concentration of quats for optimal efficacy, which could be more than double the normal concentration.
- Quaternary ammonium compounds (quats) can be applied by spraying or wiping onto certain non-food-contact surfaces and left to air dry. However, due to their tendency to adhere to surfaces, they can leave behind a residue that poses potential health and environmental hazards. Therefore, a typical cleaning process involving quats consists of a two-phase approach where the surface is first cleaned with a QAC product, followed by a thorough rinse with water. Neglecting to rinse surfaces can result in the transfer of quats onto food upon contact.
- When utilising quats for immersion sanitation of utensils, it is imperative to incorporate an intermediate hot rinse stage before direct contact with soaps or detergents.
Conclusion
Quaternary Ammonium Compounds, or Quaternary Ammonium Compounds, are powerful antimicrobial agents that can eliminate various microorganisms. They are commonly used in disinfectants and sanitisers due to their efficacy and safety for metal equipment and surfaces. Some popular QACs include benzalkonium chloride, dodecylbenzene sulfonic acid, and cetyltrimethylammonium chloride. Unlike bleach or chlorine solutions, QACs are odourless, colourless, and non-corrosive. To optimise their performance, QACs should be used in a slightly acidic to neutral pH range, typically between 6 and 8. Lastly, it’s crucial to be mindful of the considerations and follow safety guidelines when using QAC-based products.