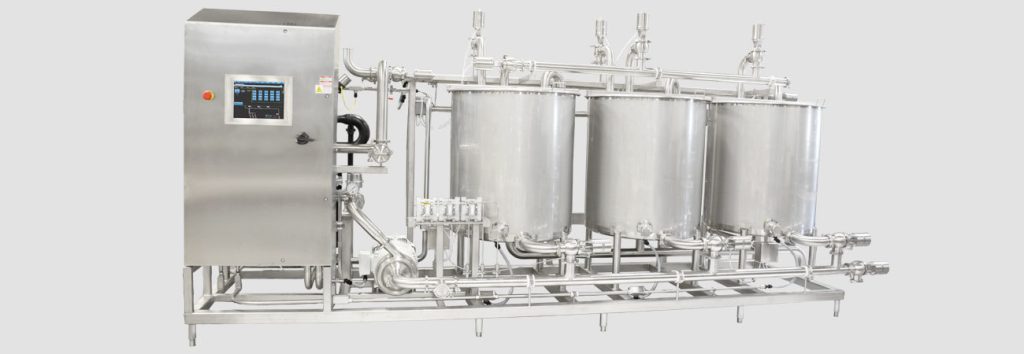
Clean in Place (CIP) is revolutionising the way equipment is cleaned in the food and beverage industry. The process allows for efficient cleaning of the interior surfaces of pipes, tanks, fillers, and other equipment without the need for disassembly. This means that operations can continue uninterrupted, saving both time and money.
The magic of CIP lies in its use of turbulent flow and spray balls to reach every nook and cranny of the equipment being cleaned. The process begins with the installation of tank cleaning machines inside the tanks. From there, a sequence of cleaning steps using water and chemicals is initiated, all of which are managed by the CIP system without the need for human intervention. This increases efficiency as well as ensures a thorough and consistent cleaning every time.
Click here for part 2.
What is Cleaning-In-Place (CIP)?

Clean-in-Place (CIP) is revolutionising the way equipment is cleaned in the food and beverage industry. The process allows for efficient cleaning of the interior surfaces of pipes, tanks, fillers, and other equipment without the need for disassembly. This means that operations can continue uninterrupted, saving both time and money.
The magic of CIP lies in its use of turbulent flow and spray balls to reach every nook and cranny of the equipment being cleaned. The process begins with the installation of tank cleaning machines inside the tanks. From there, a sequence of cleaning steps using water and chemicals is initiated, all of which are managed by the CIP system without the need for human intervention. This increases efficiency as well as ensures a thorough and consistent cleaning every time.
Where is Cleaning-In-Place (CIP) Used?

Major industries using CIP include food, dairy, and beverage, breweries, and pharmaceuticals. The CIP process is important in these industries because it helps prevent the growth of bacteria and other contaminants that could potentially harm consumers. For example, in breweries, leftover beer, yeast, and other unwanted substances can not only cause germs and bacteria to proliferate but also affect the taste of the beer. The same goes for F&B industries. As for pharmaceuticals, the CIP process helps improve the quality and efficacy of the drugs produced by removing any residual substances from previous batches.
Purpose of Cleaning-In-Place (CIP)

The Clean-In-Place (CIP) process is essential in ensuring that the product remains pure, high-quality, and safe for consumption. This process is fundamental in the event of product contamination, which can severely affect the product’s taste and efficacy, and in some cases, pose a threat to the consumers’ health. By using CIP, cross-contamination between products can be prevented, and the ingression of one product into another can be avoided.
In addition to maintaining product purity, the CIP process has several other benefits. For example, it helps to maximise equipment uptime and production capacity, allowing for the quickest possible resumption of production after a batch has been completed, which, in turn, allows for better control over the production process, ensuring that the final product meets the desired quality standards. Overall, the CIP process plays a crucial role in maintaining product quality, as well as optimising production processes.
Benefits of Using Cleaning-In-Place (CIP)

PRODUCT SAFETY: As we mentioned before, the CIP process warrants product safety by cleaning every crack and crevice of the equipment, preventing the ingression of one product into another. Therefore the reduced cross-contamination of products also guarantees increased product safety. And while this key benefit cannot be overlooked, another equally important benefit of increased employee safety also deserves special mention.
EMPLOYEE SAFETY: Trust us when we say that we have witnessed many cases of employees accidentally coming in contact with leftover chemicals leading to hospitalisations. The Clean-In-Place (CIP) process helps to guarantee employee safety by reducing the risk of exposure to contaminated products or dangerous chemicals. During the CIP process, the production equipment is thoroughly cleaned and sanitised, removing any residue or contaminants that may have accumulated during the production process. In addition, the cleaning solutions used during the CIP process are carefully selected to ensure that they are safe for use in a food production environment. These solutions are also typically monitored and regulated to meet the required safety standards, reducing the risk of employees coming into contact with contaminated products, which could potentially cause harm to their health.
INCREASED PRODUCTIVITY: The regular cleaning and sanitation of equipment help to reduce downtime, allowing for faster and more efficient production. This, in turn, leads to increased productivity, lower operating costs, and higher profits. Additionally, companies can increase customer satisfaction by producing high-quality products, leading to repeat business and a stronger bottom line.

RULES & REGULATIONS: The CIP process helps companies comply with regulations related to food safety, such as the Hazard Analysis and Critical Control Points (HACCP) program, which takes a systematic approach to food safety and focuses on identifying and controlling potential food safety hazards at different stages of the food production process. The program is based on the principle that food safety hazards can be prevented or reduced to acceptable levels by applying control measures at specific points (Critical Control Points (CCP)) in the food production process. By doing so, companies can avoid costly fines and penalties for non-compliance and protect their reputation by producing safe and high-quality products.
EVOLUTION OF CIP: The CIP process has evolved significantly over the years, with advancements in technology and increased regulations driving the development of more efficient and effective cleaning methods. In the past, CIP processes often involved more steps, with production lines having to be shut down for extended periods for cleaning. Today, however, CIP processes are highly automated and can be completed much faster, allowing for minimal downtime and increased production efficiency.
Cleaning in Place Solutions

Check out the product suggestions we’ve listed below for the Cleaning-In-Place (CIP) process recommended by ORAPI. But remember, ORAPI is focused on providing solutions, not just products. So stay tuned for part 2 of our CIP article, where we’ll dive deeper into the chemical solutions used, the CIP system structure, the step-by-step CIP cycle, and how to confirm the effectiveness of the process.
ORAPI RECOMMENDS:
SPECTRUM is a powerful alkaline cleaner designed for efficient removal of carbon buildup, rust, and grease during cleaning operations.
ORAPI RECOMMENDS:
ORAPI RECOMMENDS:
Conclusion
In conclusion, Cleaning-In-Place (CIP) is a crucial process for the food and beverage, brewery, and pharmaceutical industries. It allows for the efficient cleaning of the interior surfaces of equipment without disassembly, resulting in uninterrupted operations and cost savings. CIP is vital in ensuring product purity, preventing cross-contamination and maintaining product quality. In addition, it enhances employee safety by reducing the risk of exposure to dangerous chemicals and helps companies comply with food safety regulations. With technological advancements, the CIP process has become more efficient and automated, resulting in increased production efficiency and profitability. In the second part of this article, the focus will be on the anatomy of cleaning-in-place systems, CIP cycles, utility components of CIP and more.