What Is the Hardness of Water
In scientific terms, water hardness is generally the dissolved calcium and magnesium concentration in water. But in layperson’s terms, you may notice water hardness when your drinking glasses at home become less than crystal clear or when your hands still feel slimy after washing with soap and water. This is because hard water does not lather when it interacts with soap due to the presence of soluble bicarbonates, chlorides and sulphates of calcium and magnesium.
Measure of Hardness of Water
Hard water arises from the presence of calcium and magnesium compounds, along with various other metal elements. A general framework for classifying water hardness is as follows: water containing 0 to 60 mg/L (milligrams per litre) of calcium carbonate is categorised as soft; water with 61 to 120 mg/L is considered moderately hard; water with 121 to 180 mg/L falls into the hard category; and water exceeding 180 mg/L is classified as very hard.
Alkaline vs Acidic Solutions and Hard Water
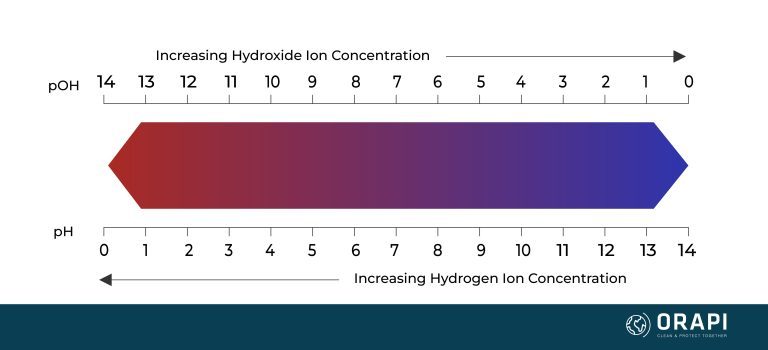
Hard Water and Alkaline Solutions
Hard Water and Acidic Solutions
Types of Hardness of Water

Temporary Hardness in Water
The presence of magnesium and calcium carbonates in water makes it temporarily hard. In this case, the hardness in water can be removed by the following methods.
How to Remove Temporary Hardness of Water
In both methods mentioned below, the goal is to convert the soluble bicarbonate compounds into insoluble carbonate compounds, which can then be physically separated from the water through filtration. By removing these insoluble compounds, the hardness of the water is reduced, resulting in water that is less likely to cause scale buildup in pipes and appliances. It’s important to note that these methods only temporarily remove water hardness, as the removed compounds can precipitate again over time.
Boiling Method
Clark's Method
Permanent Hardness in Water
When water contains the soluble magnesium and calcium salts in the chloride and sulphate forms, it is termed permanent hardness. This type of hardness cannot be removed through boiling.
How to Remove Permanent Hardness of Water
In all three methods mentioned below, the goal is to remove the calcium and magnesium ions that contribute to water hardness. These methods rely on chemical reactions or adsorption processes to reduce the concentration of these ions, resulting in softened water that is less likely to cause scale buildup and other issues associated with hard water.
Gan's Permutit Method
Calgon's Process
In the Calgon process, sodium-hexa-meta-phosphate (NaPO3)6, commonly known as Calgon, is used. Calgon works by adsorbing (adhering to the surface) calcium ions (Ca²⁺) and magnesium ions (Mg²⁺) present in the water. This reduces the concentration of these ions and thus reduces water hardness. The process involves the adsorption of Ca²⁺ and Mg²⁺ ions by the Calgon particles.
Ion Exchange Resin Method
In this method, ion exchange resins remove permanent hardness from water. Ion exchange resins are specially designed materials with functional groups that can reversibly exchange ions. In water softening, cation exchange resins remove calcium and magnesium ions. In contrast, anion exchange resins are used to remove sulphate ions.
For cation exchange:
Ca²⁺/Mg²⁺ + 2RCOOH → (RCOO)2Ca/Mg + 2H⁺
For anion exchange:
RNH2OH + Cl⁻ → RNH2Cl + OH⁻
H⁺ + OH⁻ → H2O
In the cation exchange, the resin exchanges hydrogen ions (H⁺) for calcium and magnesium ions, removing these ions from the water. In the anion exchange, the resin removes chloride ions (Cl⁻) and hydroxide ions (OH⁻) to maintain electroneutrality.
Problems Caused by Hard Water

Laundry Issues
Hard water doesn’t lather well with soap, making it less effective for laundry purposes. This can lead to poor cleaning of clothes.
Stiffness
Hard water contains high levels of minerals like calcium and magnesium, which can deposit onto fabric fibres during washing. This mineral buildup can make clothes feel stiff and rough. Many like to use fabric softeners to combat the stiffness that results from hard water usage.
Dulling of Colors
The minerals in hard water can also react with laundry detergents, forming a residue that dulls the colours of clothes over time.
Soap Scum
Hard water can prevent detergents from lathering properly, leading to soap scum buildup on clothes. This can make fabrics appear dingy and feel less clean.
Reduced Cleaning Effectiveness
Hard water can reduce the effectiveness of laundry detergents by interfering with their ability to remove dirt and stains from clothes.
Skin Irritation
Hard water can strain the skin and hair, leading to dryness and irritation for those who use it for bathing.
Increased Water Appliance Workload
Appliances like water heaters, dishwashers, and washing machines have to work harder when using hard water, potentially increasing water bills and energy consumption.
Spots on Clothes and Linens
Hard water can cause mineral deposits to form on clothes and linens, leaving behind unsightly spots.
Formation of Scales
Boiler Efficiency Reduction
Hard water can lead to the deposition of salts in boilers, reducing their efficiency over time.
Equipment Efficiency Reduction
Mineral deposits, scale or limescale, can accumulate on equipment surfaces and pipelines. This reduces the efficiency of heat exchangers, pumps, and other components, increasing energy consumption.
Toilet Bowl Deposits
Hard water can lead to mineral deposits accumulating in toilet bowls, resulting in the formation of limescale.
Steel, Copper, & PVC Pipes
As steel pipes near the end of their lifespan, calcium and magnesium buildup, known as lime scale, will increasingly obstruct them. This problem isn’t limited to steel pipes alone; PVC and copper pipes can also become blocked over time due to mineral deposits. Signs of this issue include reduced water pressure and the presence of particles in your faucet or shower water. Moreover, rising pipe pressure can lead to cracks forming, causing water to divert to other areas of your home before reaching the desired sink or fixture.
Wastage
Soap Scum
The reaction between hard water and soap can lead to the formation of scum, wasting soap and making cleaning less effective.
Excessive Fuel Consumption
Industries that use hard water for processes may need more fuel to sustain extended and intensified machine operation due to the increased workload caused by hard water.
Common Signs of Hard Water
- Linens and garments lose their lustre and exhibit a coarse texture.
- Unattractive blemishes on white ceramic surfaces accompanied by mineral deposits on faucets.
- Reduced shower water pressure resulting from obstructed pipelines.
- Formation of chalky, white marks or patches on dishware.
- Visible streaks and marks emerge within the shower area.
How to Treat Hard Water

Water Softener
Water Conditioner
Water conditioners utilise template-assisted crystallisation (TAC) media to crystallise the minerals responsible for water hardness. This process effectively inhibits the accumulation of scale within pipes and appliances. Unlike water softeners that employ ion exchange resin to extract these minerals from water, water conditioners bring about physical alterations to the minerals, thereby impeding their ability to cause scaling issues.
Conclusion: What Is Water Hardness
In conclusion, understanding the intricacies of water hardness is crucial for optimising the use of this vital resource. While minerals like calcium and magnesium are essential for health, their excessive presence in water can lead to various challenges, affecting household and industrial applications. This article has delved into the concept of water hardness, its effects on cleaning efficiency, and methods for its removal. From temporary to permanent hardness, the implications of hard water encompass laundry woes, skin irritation, equipment inefficiencies, and more. By comprehending the nuances of water hardness, we can make informed decisions to ensure efficient, sustainable, and effective utilisation of water resources in various contexts.