Rust is the nemesis of metal, a relentless force that can compromise the integrity and appearance of everything from industrial machinery to household tools. While removing rust is a critical step in metal maintenance, it is equally important to prevent its return. This is where passivation comes into play. Passivation is a process that enhances the corrosion resistance of metals, particularly stainless steel, by forming a protective oxide layer on the surface. In this article, we’ll explore what passivation is, how it works, and how you can use it to prevent rust after removal.

History of the Passivation Process
The concept of passivation has been around for centuries, with early observations dating back to the 19th century. Scientists discovered that certain metals, particularly stainless steel, could develop a natural oxide layer that protected them from corrosion. This phenomenon was formally studied in the early 20th century, leading to the development of controlled chemical passivation techniques.
During World War II, passivation became a crucial process in the aerospace and military industries, as the need for corrosion-resistant materials grew. By the mid-20th century, industrial standards and regulations were established, defining best practices for passivation in various industries. Today, advancements in chemistry and materials science have expanded passivation applications beyond stainless steel to include other metals and advanced coatings.
What is Passivation?
Passivation is a chemical process that removes free iron and other contaminants from the surface of a metal, typically stainless steel, while promoting the formation of a thin, inert oxide layer. This layer acts as a barrier, shielding the metal from environmental factors that cause corrosion, such as moisture, oxygen, and chemicals.
The process is especially important for stainless steel, which derives its corrosion-resistant properties from the presence of chromium. When exposed to oxygen, chromium reacts to form chromium oxide, a passive layer that prevents further oxidation. When properly executed, it significantly improves the durability and longevity of metal surfaces.
Why is Passivation Necessary After Rust Removal?
When rust is removed from a metal surface, the underlying material is exposed and often left in a reactive state. Without proper treatment, the metal is prone to rapid re-oxidation, especially in humid or corrosive environments. Passivation ensures that the surface is not only clean but also chemically stable, reducing the likelihood of rust reformation.
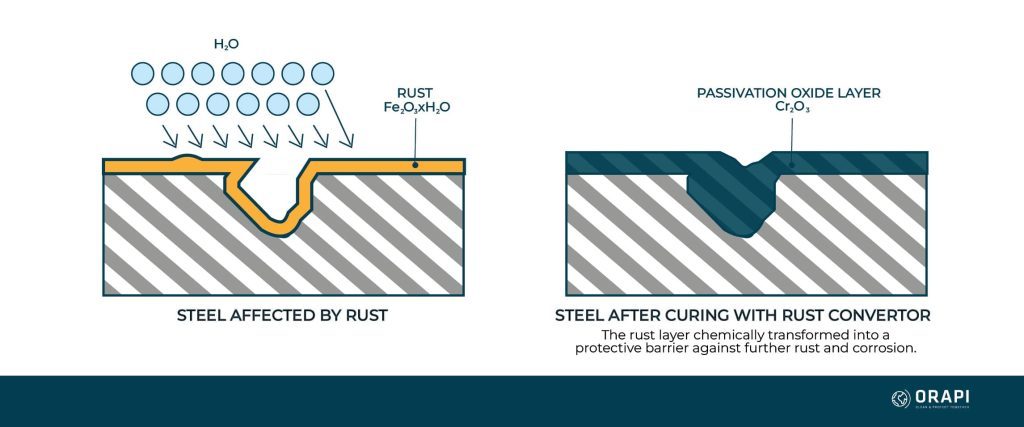
For example, after mechanically or chemically removing rust from stainless steel, microscopic iron particles may remain on the surface. These particles can act as initiation points for new rust formation. Passivation removes these contaminants and allows the natural chromium oxide layer to regenerate, providing long-term protection.
Passivation Types
There are various types of passivation processes, each suited for different metals and applications:
- Nitric Acid Passivation – One of the most common methods, nitric acid removes free iron while promoting chromium oxide formation. It is effective but can be hazardous due to its strong oxidizing properties.
- Citric Acid Passivation – A safer and more environmentally friendly alternative, citric acid effectively removes iron contaminants while being biodegradable and non-toxic.
- Electrochemical Passivation – Uses an electric current in an acid solution to accelerate the passivation process, providing enhanced control and efficiency.
- Thermal Oxidation Passivation – Utilizes controlled heating to form a protective oxide layer, often used in high-temperature applications.
- Formic Acid Passivation – commonly used in High-Performance Liquid Chromatography (HPLC) as an alternative to traditional nitric or citric acid passivation. It provides a safer and more environmentally friendly option. The corrosiveness of formic acid increases with the dissociation of H⁺ ions, which helps activate the passivation process and enhances its effectiveness in removing surface contaminants.
Benefits of Passivation
- Enhanced Corrosion Resistance: Strengthens the metal’s natural oxide layer to prevent rust.
- Extended Lifespan: Protects structural integrity, reducing replacement costs.
- Improved Aesthetic Appeal: Creates a clean, uniform surface free of discolouration.
- Improved Paint Adhesion: A passivated surface provides a clean, stable foundation for paints and coatings. By removing contaminants and creating a uniform oxide layer, passivation ensures better bonding of paints, reducing peeling or blistering over time. This makes it an ideal preparatory step for applications requiring both corrosion resistance and a painted finish.
- Cost-Effective Maintenance: Reduces the need for frequent repairs.
The Key Drawback of Passivation
While passivation offers numerous benefits, it is not without limitations. One significant drawback is that passivation is not a permanent solution. Over time, the passive layer can degrade due to mechanical wear, harsh chemicals, or environmental factors. For example, in high-chloride environments (e.g., coastal areas), the layer may break down faster.
Additionally, passivation is only effective on metals that naturally form a protective oxide layer, such as stainless steel. It is unsuitable for carbon steel or iron, which lack sufficient chromium. In such cases, coatings or galvanization are better options. However, with newer technology in polymer emulsion, advanced rust converters can now chemically transform rust into a durable, protective layer, offering an alternative to traditional passivation in certain applications.

The Passivation Process
Before applying the passivator, prepare the surface to obtain the best results:
- Wire Brush: Remove loose rust and paint flakes from the surface (no sanding required).
- Degrease the Surface: Use a harmless solvent such as ORASOLV T to degrease the surface.
- Apply Passivator: Apply the passivator directly onto the rusted surface using a brush or spray. For optimal results, apply two thin coats. Avoid dipping the brush directly into the master container, as it can damage the remaining product.
- Drying: The passivator will turn black and dry to the touch in about 15 minutes. You can apply a second coat within 1-2 hours.
- Curing Time: Allow the product to dry for 16 to 24 hours before applying solvent-based paint.
- Clean Up: Clean the brush and any spillage with ORASOLV T and water while the passivator is still wet.
Passivation with Polymer Emulsion
Another effective method for enhancing corrosion resistance is using polymer emulsions in passivation. Polymer emulsions create a thin, durable film on the metal surface, acting as an additional barrier against moisture and contaminants. This approach is particularly useful for metals that undergo frequent handling or exposure to harsh environments.
How Polymer Emulsion Works in Passivation:
- Surface Preparation: The metal is cleaned and prepped similarly to standard passivation methods.
- Application of Polymer Emulsion: A specialized polymer-based coating is applied, forming a flexible, corrosion-resistant film.
- Curing and Drying: The emulsion dries and bonds to the metal, enhancing the protective oxide layer and providing long-term durability.
- Improved Protection: The combination of passivation and polymer emulsion extends corrosion resistance beyond traditional methods, making it ideal for demanding industrial applications.
Tips for Effective Passivation
- Choose the Right Chemical: Traditional rust removal methods often rely on acids like nitric acid, but modern rust converters with polymer emulsion properties, such as BLOC RUST, are becoming a preferred choice. These advanced formulations chemically transform rust into a stable, protective layer that prevents further corrosion while also serving as a primer for coatings or paint. Their ease of application, improved safety, and environmental benefits make them ideal for industrial, automotive, and marine applications. When selecting a rust converter, ensure proper surface preparation, application method, and drying time to maximize effectiveness and longevity.
ORAPI RECOMMENDS:
BLOC’RUST is a powerful rust passivator which chemically transforms rust into a tough, protective polymer coating. It stops rust before it becomes a major problem.
- Follow Manufacturer Guidelines: Different metals and alloys require specific passivation procedures, so always follow the manufacturer’s recommendations. At ORAPI, we provide both Safety Data Sheets (SDS) and Technical Data Sheets (TDS) to ensure proper usage. Download our SDS and TDS for detailed guidelines.

- Avoid Contamination: Use clean, deionized water for rinsing to prevent the introduction of new contaminants. Handle with Care: Wait for the surface to dry completely to allow the passivation process to take full effect.
- Regular Maintenance: Even passivated surfaces can benefit from periodic cleaning with cleaning solvent and inspection to ensure ongoing protection.
ORAPI RECOMMENDS:
ORASOLV S1 is a multi-purpose high technology biodegradable solvent replacing chlorinated and cetonic solvents in some applications. Guaranteed hydrocarbons free.
Passivation Specifications Standard
Knowing industry standards, such as passivation specifications, is crucial for ensuring the quality, durability, and corrosion resistance of metal components, particularly in industries like aerospace, medical devices, and manufacturing. These standards, such as ASTM A967, AMS 2700, and ISO 13485, provide guidelines on proper chemical treatments, testing methods, and acceptance criteria for passivated surfaces. Adhering to these specifications helps maintain compliance with regulatory requirements, improves product performance, and enhances customer confidence. Additionally, understanding these standards allows manufacturers to prevent contamination, extend the lifespan of components, and reduce costly rework or failures in critical applications.
Industry Standards – Passivation Specifications
If you’re looking for a stainless steel passivation specification, there are several industry standards that define the process.
|
Standard |
Title / Description |
|
Standard Specification for Chemical Passivation Treatments for Stainless Steel Parts
|
|
|
Passivation of Corrosion Resistant Steels
|
|
|
Standard Practice for Cleaning, Descaling, and Passivation of Stainless Steel Parts, Equipment, and Systems
|
|
|
(superseded) Passivation Treatments for Corrosion-Resistant Steel
|
|
|
Standard Practice for Surface Preparation and Marking of Metallic Surgical Implants |
|
|
Standard Practice for Permanent Marking of Orthopaedic Implant Components |
|
|
Standard Guide for Descaling and Cleaning Titanium and Titanium Alloy Surfaces
|
|
|
Corrosion-Resistant Steel Parts: Sampling, Inspection and Testing for Surface Passivation |
|
|
Aerospace Series: Passivation of Corrosion Resisting Steels and Decontamination of Nickel Base Alloys |
Military Specs and Standards
|
Standard |
Refers to |
Title / Description |
|
QQ-P-35 MIL-STD-753 |
Finish, Protective and Codes for Finishing Schemes for Ground and Ground Support Equipment:
|
|
|
ASTM A380 |
Finishes for Ground-Based Electronic Equipment:
|
|
|
ASTM A967 AMS 2700 |
Surface Treatments and Inorganic Coatings For Metal Surfaces of Weapons Systems:
|
|
|
ASTM A967 AMS 2700 ASTM A380 |
Finishing of Metal and Wood Surfaces:
|
Conclusion
Rust removal is only half the battle; preventing its return is equally crucial. Passivation is a proven method for enhancing the corrosion resistance of metals, particularly stainless steel, by restoring and strengthening their natural protective oxide layer. By understanding and implementing the passivation process, you can safeguard your metal components against rust, ensuring their durability and performance for years to come. Whether you’re working with industrial equipment, automotive parts, or construction, passivation is a key step in maintaining the integrity and appearance of your metal surfaces.