Corrosion poses a significant threat to the integrity and safety of metal structures, particularly in critical applications like aviation maintenance, repair, and overhaul (MRO). Understanding corrosion forms is essential for MRO professionals, as it enables them to implement adequate preventive measures and inspection protocols, ensuring the longevity, safety, and reliability of aircraft components, where various types of corrosion in aircraft can be a significant threat to the integrity and safety of metal structures.
This article explores the various manifestations of corrosion, including types of corrosion in aircraft, to provide valuable insights for MRO professionals.
Types of Corrosion

Two primary categories encompass the majority of corrosion manifestations: direct chemical deterioration and electrochemical deterioration. In both forms of corrosion, the metal transforms into a metallic compound, such as an oxide, hydroxide, or sulphate. This corrosion process entails two concurrent transformations: the metal subject to attack or oxidation experiences an anodic alteration, while the corrosive agent undergoes reduction, constituting a cathodic transformation.

Forms of Corrosion in Aircraft

Corrosion manifests in various forms, with the specific type depending on factors such as the metal’s composition, dimensions, function, prevailing atmospheric conditions, and the presence of corrosive agents, making it crucial to consider types of corrosion in aircraft.
Surface Corrosion
General surface corrosion is one of the most common types of corrosion in aircraft, also known as uniform etch or uniform attack corrosion. This type of corrosion causes the surface of a metal to become roughened, etched, or pitted and often leaves behind a powdery deposit of corrosion products. Surface corrosion can occur due to either direct chemical or electrochemical attack. In some cases, the corrosion may spread under the surface coating, making it difficult to detect through roughening or powdery deposits. Closer inspection may reveal small blisters in the paint or plating caused by the pressure of the underlying accumulation of corrosion products.
Filiform Corrosion

Among the different types of corrosion in aircraft filiform corrosion is a unique because of oxygen concentration that develops on metal surfaces with an organic coating system. This type of corrosion can be identified by the distinctive worm-like pattern of the corrosion products that appear beneath the paint film.
Pitting Corrosion
Pitting corrosion falls under one of the most destructive types of corrosion in aircraft that can affect metals like aluminum and magnesium alloys, leading to small holes or pits on the surface and causing disproportionate damage to structural components.. It initially appears as a white or grey powdery deposit that resembles dust and covers the surface. Upon removal of the deposit, small holes or pits can be observed on the surface. These seemingly insignificant openings can penetrate into structural components and cause disproportionate damage to their surface appearance.
Dissimilar Metal Corrosion
Extensive pitting damage can occur when dissimilar metal components come into contact in the presence of a conductor. While surface corrosion may or may not be evident, a galvanic process, similar to electroplating, takes place at the points where the insulation between the surfaces has deteriorated or is absent. This electrochemical attack can pose a significant threat because, in many cases, it remains hidden from view, and the only way to identify it before structural failure is through disassembly and inspection.
Mechanical contamination of a metal’s surface can also lead to dissimilar metal corrosion. Inappropriate use of steel cleaning products, such as steel wool or a steel wire brush on aluminium or magnesium, can introduce small steel particles into the cleaned metal, resulting in corrosion and damage to the adjacent surface. It is crucial to exercise caution when using non-woven abrasive pads to ensure that pads used on one type of metal are not subsequently used on a different metal surface.
Concentration Cell Corrosion
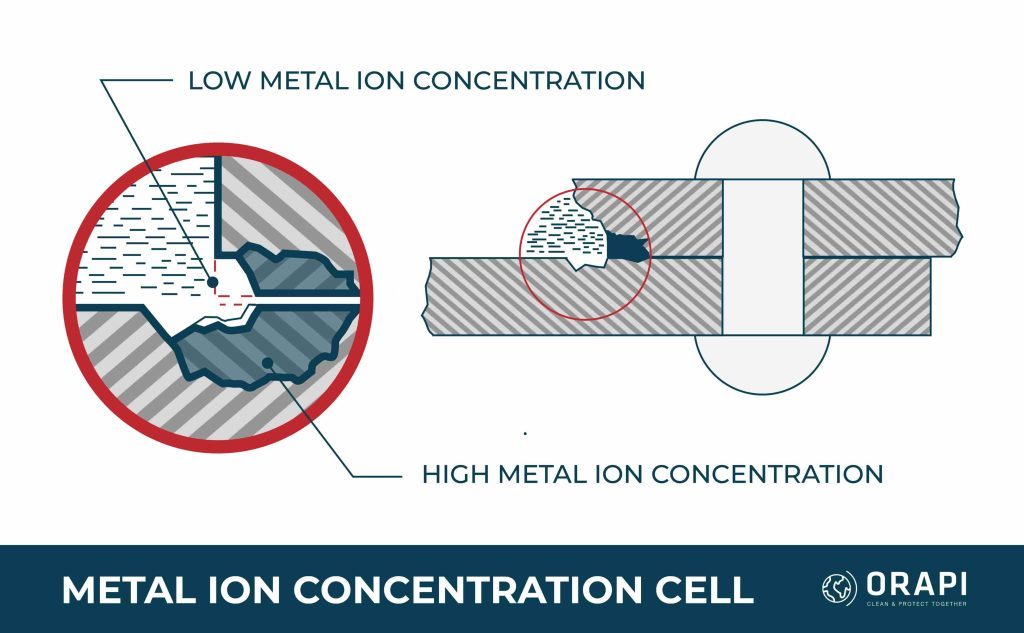
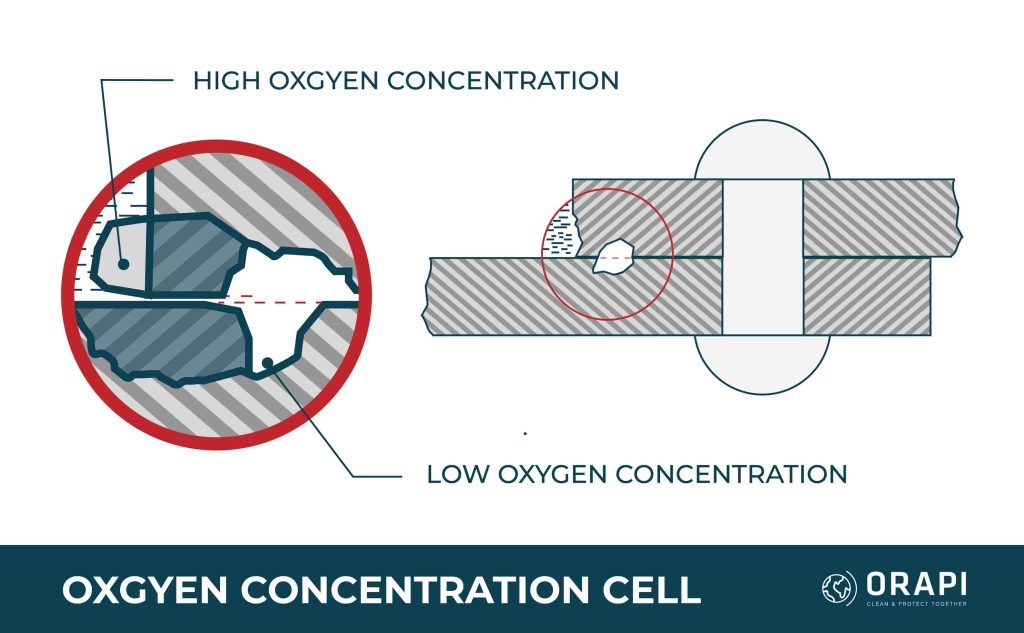
Corrosion that occurs in a metal-to-metal joint, at the edge of a joint despite the joined metals being the same, or on a spot of metal surface covered by foreign material is known as concentration cell corrosion, also called crevice corrosion. Three main types of concentration cell corrosion are metal ion concentration, oxygen concentration, and active-passive cells.
Metal Ion Concentration Cells
The most destructive and intense type of corrosion is pitting corrosion, which can affect any metal but is more commonly found on metals that form protective oxide films, such as aluminium and magnesium alloys. It initially appears as a white or grey powdery deposit that resembles dust and covers the surface. Upon removal of the deposit, small holes or pits can be observed on the surface. These seemingly insignificant openings can penetrate into structural components and cause disproportionate damage to their surface appearance.
Oxygen Concentration Cells
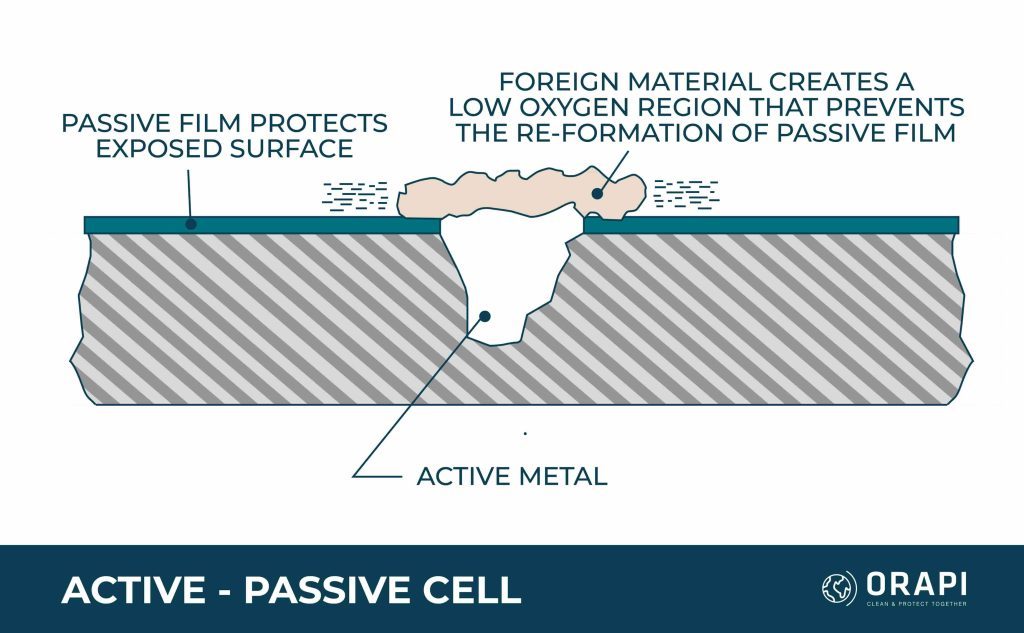
Active-Passive Cells
Certain metals rely on a firmly attached passive film, typically an oxide, to protect against corrosion, but they are susceptible to quick corrosive damage from active-passive cells. The corrosive process usually initiates as an oxygen concentration cell. The dirt particle breaks through the passive film, exposing the active metal to corrosive harm. This creates an electrical potential between the passive film’s extensive area and the active metal’s limited area, leading to rapid pitting.
Intergranular Corrosion
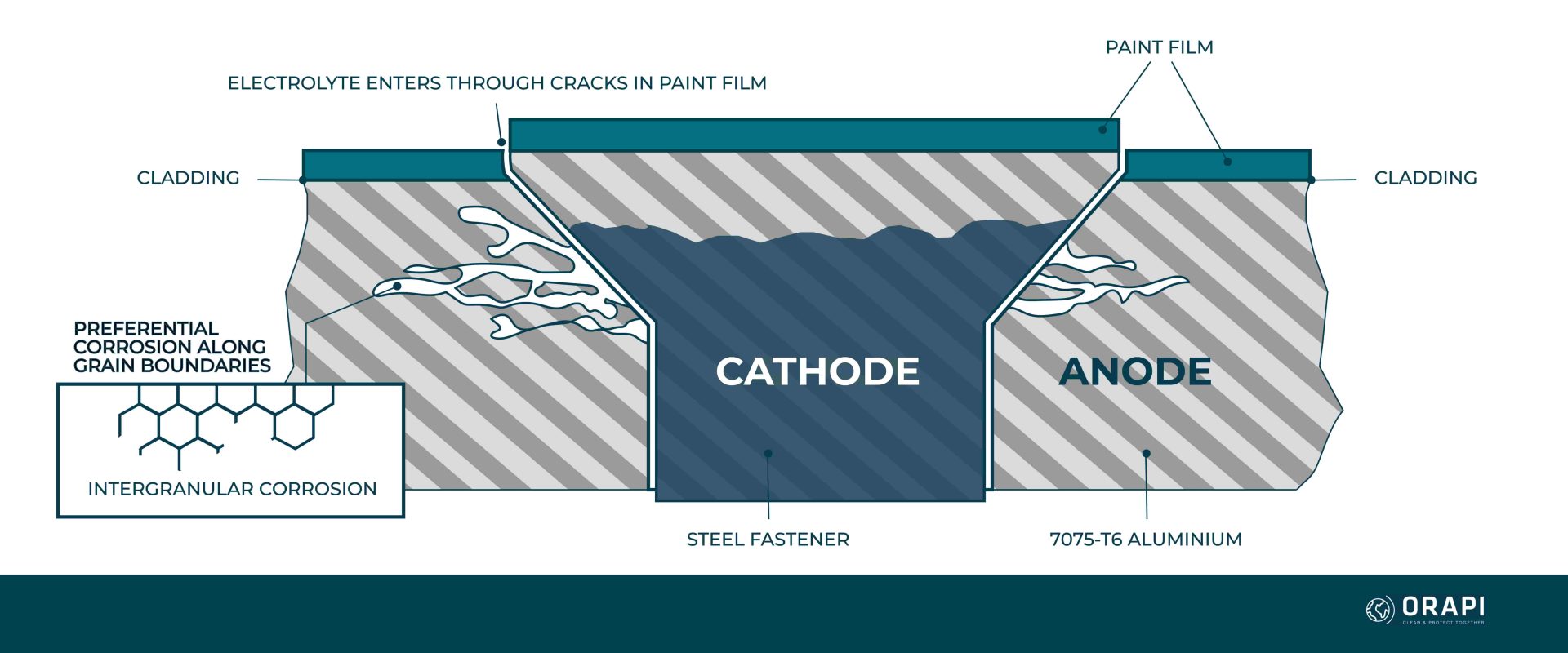
Intergranular corrosion is one of the types of corrosion of an aircraft that is characterised by its occurrence along the grain boundaries of an alloy, often arising due to structural non-uniformity within the alloy. This type of electrochemical deterioration is notably prevalent in aluminium alloys and certain stainless steels. The non-uniformity in the alloy structure is primarily induced by the thermal fluctuations experienced during the manufacturing process. Notably, intergranular corrosion can be present without visible indications on the material’s surface. When it comes to high-strength aluminium alloys, such as 2014 and 7075, their vulnerability to intergranular corrosion is heightened when they have undergone improper heat treatment and subsequently been exposed to a corrosive environment.
Exfoliation Corrosion
Exfoliation corrosion represents an advanced stage of intergranular corrosion, characterised by the elevation of surface grains in a metal due to the expansion of corrosion products near the grain boundaries just beneath the surface. This phenomenon is visible proof of intergranular corrosion and is predominantly observed in extruded sections, where grain thickness typically falls below rolled forms. Detecting this form of corrosion in its early stages is challenging. Extruded components, including spars, can be susceptible to exfoliation corrosion. Effective inspection techniques involve using ultrasonic and eddy current methods, which have demonstrated significant success in identifying and monitoring this corrosion type.
Stress Corrosion Cracking
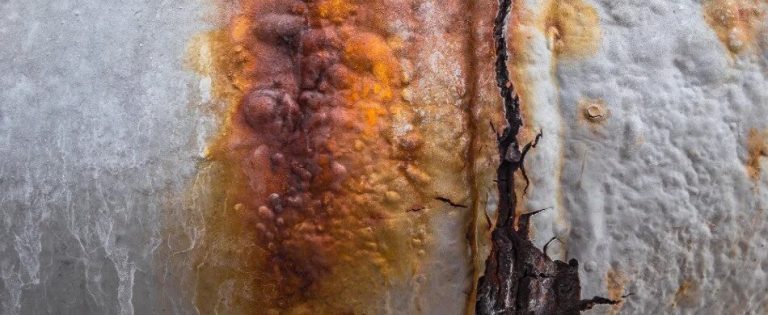
This form of corrosion is characterised by constant or cyclic stress, which interacts with a corrosive chemical environment. This stress can originate from either internal or external sources. Internal stress may become embedded in a structure during manufacturing processes like cold-working or due to uneven cooling from high temperatures.
Manufacturers typically include a stress relief operation in their processes to alleviate internal stress. Nevertheless, there are instances where stress remains trapped within the structure. External stress may also be introduced into the structure through riveting, welding, bolting, clamping, or press fitting. Even slight mismatches or over-tightening of fasteners can lead to internal stress. Internal stress takes precedence over design stress in terms of significance because stress corrosion is often challenging to detect until it surpasses the design safety margin. Stress levels can vary from one point to another within the metal, with stresses near the yield strength generally being required to initiate stress corrosion cracking. However, failures may occur at lower stress levels.
Various conditions have been identified that lead to stress corrosion cracking in specific types of alloys:
- High-strength, heat-treated steel and aluminium alloys are susceptible to stress corrosion cracking when exposed to salt solutions and seawater.
- Some titanium alloys can experience stress corrosion cracking when exposed to solutions containing methyl alcohol and hydrochloric acid.
- In moist air environments, magnesium alloys may undergo stress corrosion.
Several strategies can be employed to mitigate stress corrosion, including applying protective coatings, subjecting materials to stress relief heat treatments, using corrosion inhibitors, and controlling the surrounding environment. Another effective method is shot peening, which enhances resistance to stress corrosion cracking by inducing compressive stresses on the surface. These compressive stresses must be overcome by applied tensile stress before any surface tension load is encountered, thereby increasing the threshold stress level.
Fretting Corrosion
Fretting corrosion represents a highly destructive corrosive attack, manifesting when two adjoining surfaces, typically in a state of relative rest, experience minimal relative motion. This phenomenon is marked by the formation of pits on the surfaces and the substantial production of finely fragmented debris. Due to the constrained movement of these surfaces, the debris is hindered from escaping easily, leading to an extremely localised abrasive effect. The presence of water vapour notably exacerbates this form of deterioration. In cases where the contact areas are small and pointed, it can result in the development of deep grooves resembling Brinell marks or pressure indentations on the contacting surface.
Consequently, this corrosion affecting bearing surfaces is also called false Brinelling. An illustrative instance of fretting corrosion can be observed in “smoking rivets” on aircraft engine cowling and wing skins. Intriguingly, this corrosion reaction is not driven by an electrolyte, and, in fact, moisture may hinder the process. A smoking rivet is distinguishable by a black ring encircling the rivet.
Fatigue Corrosion


When two different metals come into contact in the presence of an electrolyte, galvanic corrosion occurs. The difference influences the speed of corrosion in activities between the metals. The greater the difference in activities, the quicker the corrosion will transpire. Additionally, the rate of galvanic corrosion is affected by the size of the metal parts in contact. If the corroding metal has a smaller surface area than the less active metal, corrosion will occur quickly and severely. Conversely, the corrosion will be slow and superficial if the corroding metal has a larger surface area than the less active metal.
Conclusion: Types of Corrosion in Aircraft
In conclusion, a thorough understanding of the diverse corrosion types is paramount for maintaining metal structures, especially in critical applications like airframes. From surface corrosion to stress-corrosion cracking and galvanic corrosion, Understanding these various types of corrosion in aircraft is essential for effective maintenance and safety measures. Filiform, pitting, and concentration cell corrosion require special attention due to their unique triggers and hidden damage potential. Industries must implement preventive measures and inspection protocols to detect and mitigate corrosion. Combining protective coatings, stress relief treatments, material selection, and environmental control can enhance metal component resilience. Proactive corrosion management ensures safety and reliability in various industries.