Stain removal has always been a paramount challenge in maintaining pristine surfaces, leading to the development of specialised cleaning agents. Alkaline solutions have emerged as a powerful tool against oil-based stains, leveraging the science of saponification to emulsify and dissolve oils effectively. Conversely, mineral stains demand the acidic touch, as these solutions excel at breaking down stubborn mineral deposits. In this concise article, we uncover the chemical underpinnings of these cleaning practices and explain the reasons behind the remarkable efficacy of alkaline vs acidic cleaners in combating distinct types of stains.
The Relationship Between pH and Hydrogen
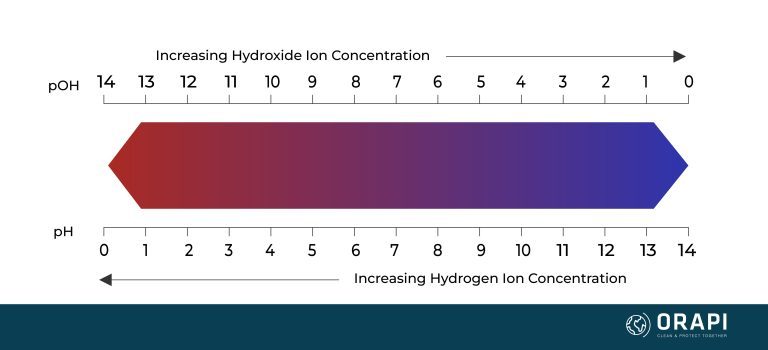
pH and hydrogen are closely related. The term pH is a measure of the acidity or alkalinity of a solution and is determined by the concentration of hydrogen ions (H+) in the solution. The pH scale ranges from 0 to 14, with values below 7 indicating acidity, values above 7 indicating alkalinity, and a pH of 7 representing neutrality.
In an aqueous solution, water molecules can undergo a process called self-ionisation, where a small fraction of water molecules dissociate into hydrogen ions (H+) and hydroxide ions (OH-). This process can be represented by the following equation:
H2O ⇌ H+ + OH-
In pure water, the concentration of hydrogen ions and hydroxide ions are equal, resulting in a neutral pH of 7. However, when other substances are dissolved in water, they can affect the balance of hydrogen and hydroxide ions, leading to either an acidic or alkaline solution.
Hydrogen and Hydroxide Ions
[H+][OH-]=Kw=10-14(=fixed) at 25℃
In any solution, the concentrations of hydrogen ions and hydroxide ions are related by a constant called the ion product of water, denoted as Kw. This constant has a fixed value of 10^-14 at 25℃ (room temperature). This means that in any solution, the product of the hydrogen ion concentration [H+] and the hydroxide ion concentration [OH-] will always equal 10^-14.
[H+]=[OH-] , [H+]=[OH-]=√(Kw)=√10-14=10-7
In pure water or a neutral solution, the concentration of hydrogen ions is equal to the concentration of hydroxide ions, which can be represented as [H+] = [OH-]. Since their product is Kw (which is 10^-14), each of their concentrations in pure water is the square root of Kw, which is 10^-7.
Therefore, in pure water, the concentration of both hydrogen ions and hydroxide ions is 10^-7 moles per litre (mol/L), and this value is used as the standard for measuring pH. The pH scale is a logarithmic scale that measures the acidity or alkalinity of a solution based on the concentration of hydrogen ions. By measuring [H+], we can determine the pH value of a solution, as pH is determined by the hydrogen-ion concentration.
Acids and Alkalis

In an acidic solution, there is an excess of hydrogen ions compared to hydroxide ions. The higher the concentration of hydrogen ions, the lower the pH value. For example, a solution with a pH of 2 has a higher concentration of hydrogen ions than a solution with a pH of 5.
In contrast, in an alkaline or basic solution, there is an excess of hydroxide ions compared to hydrogen ions. The higher the concentration of hydroxide ions, the higher the pH value. For example, a solution with a pH of 10 has a higher concentration of hydroxide ions than a solution with a pH of 8.
So, the concentration of hydrogen ions directly influences the pH value of a solution. Changes in the concentration of hydrogen ions can result in changes in pH, indicating the difference between alkaline vs acidic cleaners.
Why Are Alkalis More Effective At Removing Oils

Saponification
Alkalis, such as sodium hydroxide (NaOH) or potassium hydroxide (KOH), can undergo a process called saponification when they react with oils or fats. During saponification, the alkalis hydrolyses the ester bonds present in oils and fats, breaking them down into glycerol and fatty acid salts (soaps). These soap molecules have both polar and nonpolar ends, making them effective emulsifiers.
Emulsification
Soap molecules have a hydrophilic (water-attracting) polar head and a hydrophobic (water-repelling) nonpolar tail. When added to water containing oils, the soap molecules surround and encapsulate the oil droplets, forming structures called micelles. The nonpolar tails of the soap molecules face inward, protecting the oil from the surrounding water, while the polar heads face outward, interacting with water molecules.
Dispersion and Solubilisation
The formation of micelles allows the oil droplets to be dispersed and suspended in the water. This dispersal effect increases the surface area of the oil, making it easier for water to interact with and dissolve the oil molecules. As a result, the oil is broken down into small, dispersed particles within the micelles, which can be easily washed away with water. This is why soaps and detergents, which are derived from alkalis through the process of saponification, are widely used as potent cleaning agents to combat oily residues effectively.
Alkaline pH
Since most soils are acidic, alkaline cleaners are very effective. Alkalis typically have a higher pH value (above 7), making the environment more basic. The alkaline pH helps neutralise any acidic components present in the oil, further facilitating the emulsification and removal process.
Why Are Acids More Effective At Removing Minerals

Acids are more effective at removing mineral stains due to their ability to dissolve and break down the insoluble mineral compounds present in these stains. Mineral stains, such as limescale, rust, and hard water deposits, are often composed of minerals that are not easily soluble in water or other regular cleaning agents. However, when acids are applied to these stains, they undergo chemical reactions that facilitate the dissolution of mineral deposits.
Acid Dissolution
Acidic substances release hydrogen ions (H+) when dissolved in water. These hydrogen ions create an acidic environment that enhances the ability of the acid to interact with the mineral compounds in the stain.
Acid-Base Reactions
The presence of hydrogen ions in the acid leads to acid-base reactions with the minerals. These reactions involve the acid’s hydrogen ions reacting with the hydroxide ions (OH-) or other negatively charged ions present in the mineral compounds. This results in the formation of water and soluble salts, which can be easily dissolved and washed away.
For example, when hydrochloric acid (HCl) reacts with calcium carbonate (CaCO3) found in limescale deposits, the following reaction occurs
HCl + CaCO3 → CaCl2 + CO2 + H2O
The calcium carbonate breaks down into calcium chloride (a soluble salt), carbon dioxide gas, and water, effectively dissolving the limescale deposit.
Because of the acid-base reaction toilet bowl limescale removers are acidic in nature. Limescale, composed mainly of calcium carbonate, serves as a base and readily reacts with acids. Consequently, the chemical interaction between the acid and limescale leads to the dissolution of the mineral deposits.
Solubility Enhancement
Acids can alter the solubility of mineral compounds, making them more soluble in water. This change in solubility allows the acid to dissolve and loosen the mineral deposits from the surface they are adhering to.
Chelation
Some acids can form chelates with certain metal ions found in mineral stains. Chelation involves the acid molecules binding to metal ions, forming water-soluble complexes. This process aids in the removal of metal-based stains, such as rust.
Conclusion: Alkaline vs Acidic Cleaners
In conclusion, the efficacy of alkaline vs acidic cleaners in stain removal lies in their distinct chemical properties and interactions with different types of stains. Alkaline solutions are potent against oil-based stains, thanks to the process of saponification, which emulsifies and disperses oils in water, while also benefiting from their alkaline pH that neutralises acidic components. On the other hand, acidic solutions excel at dissolving mineral stains by engaging in acid-base reactions that break down stubborn mineral compounds into soluble salts. Understanding the specific mechanisms behind these cleaning practices empowers us to make informed choices and achieve optimal stain removal results in our cleaning endeavours. Whether tackling oil residues or mineral deposits, utilising the right cleaning agents based on their chemical properties remains key to maintaining spotless surfaces.