What is Galvanic Corrosion?
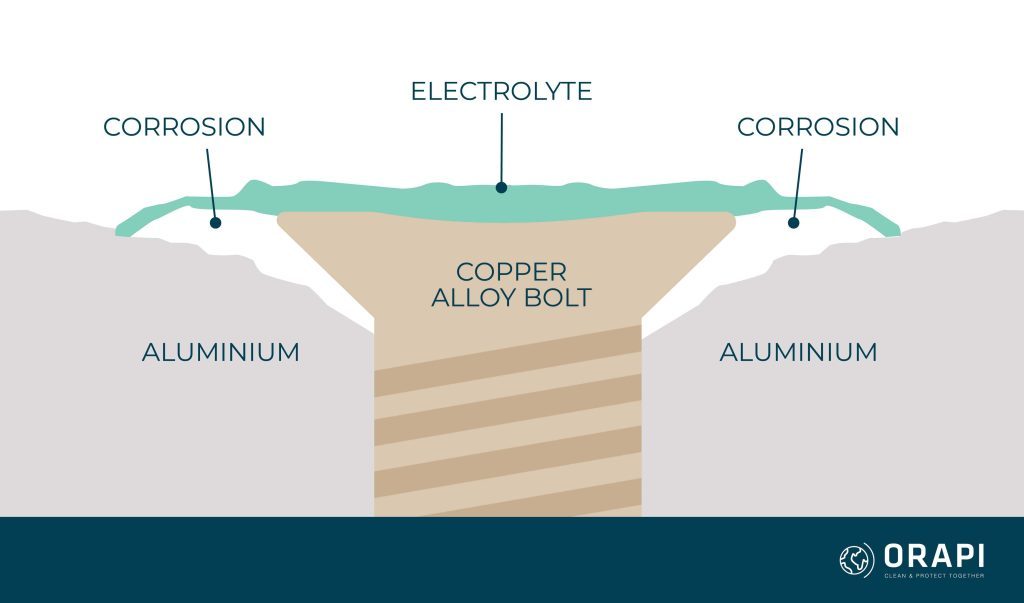
Galvanic corrosion, a relentless adversary in the world of materials and engineering, emerges as a formidable challenge when the wrong choice of metals collides. Many would find it appropriate to secure aluminium components in place with copper alloy bolts. But as is evident in the image above a white area surrounding the central copper alloy bolt appears on the adjacent aluminium. In addition, steel bolts, when improperly matched with bronze plaques, also succumb to galvanic corrosion, leaving behind unsightly rust stains. In this article, we explore the causes, effects, and the science behind galvanic corrosion and elaborate on how to prevent and treat it.
The Galvanic Series
Metals can be systematically arranged in a galvanic series, aiding the assessment of their corrosion susceptibility when they come into contact. This series, detailed in Table 1, establishes the galvanic hierarchy of various metals, mainly in seawater. Notably, though non-metallic, graphite conducts electricity well enough to induce galvanic corrosion and is included in Table 1.
The galvanic series organises metals from the most reactive (lower end) to the least reactive (top). The lower-ranked metal in the series is considered more active in pairs of metals, while the higher-ranked is more noble. When two metals contact each other, the more active metal is prone to galvanic corrosion. For example, zinc in contact with mild steel may corrode because it’s more active than mild steel.
Table 1: The galvanic series from the most noble at the top to the most active at the bottom.

|
Graphite (carbon) |
|
Platinum |
|
Gold |
|
Stainless steel (alloy 316) |
|
Silver |
|
Nickel |
|
Copper nickel (70:30 wt%) |
|
Lead |
|
Tin bronze |
|
Lead-tin solder (50:50 wt%) |
|
Tin |
|
Copper |
|
Yellow brass |
|
Chromium (plated on steel) |
|
Mild steel, cast iron |
|
Cadmium |
|
Aluminium alloys |
|
Zinc |
|
Magnesium |
Galvanic Corrosion Formation

To facilitate galvanic corrosion, two conditions must be met. Firstly, the metals involved must establish electrical connectivity, which can be achieved through direct physical contact or by using another conducting medium, enabling the passage of an electric current from one metal to the other. Secondly, they must maintain ionic connectivity, allowing an ion current to flow between them. This necessitates the presence of an electrolyte, typically a solution containing ions, such as those found in dissolved salts, acids, or bases. Ionic connectivity can be established when the metals are completely submerged in the electrolyte or when they are coated with a continuous film of electrolyte that adequately wets both metals, a scenario often observed in high humidity conditions.
The Science Underlying Galvanic Corrosion
Galvanic Series and Corrosion Potential
The phenomenon of galvanic corrosion can be understood through the science of corrosion potential and the galvanic series. When a metal is exposed to an electrolyte, it undergoes a process in which its atoms transition into ions and electrons (e-). Take, for instance, iron (Fe), which experiences the following reaction:
Fe → Fe2+ + 2e−
This is referred to as an anodic reaction, resulting in the formation of Fe2+ ions. Concurrently, other reactions extract electrons; for example, oxygen (O2) dissolved in the electrolyte can engage in a reaction to generate hydroxide ions (OH−), as follows:
O2 + 2H2O + 4e− → 4OH−
This is known as a cathodic reaction. The electrons are transferred into the metal during the anodic reaction and removed from it during the cathodic reaction. Equilibrium is attained when electrons are deposited and extracted at an equivalent rate.
The interaction between the electric charges of ions and electrons causes the metal to develop an electric potential. In the case of a corroding metal, where multiple reactions occur simultaneously, this potential is termed a mixed potential or corrosion potential. The galvanic series, exemplified in Table 1, is based on these corrosion potential values. Figure 1 presents corrosion potentials for various metals and alloys when immersed in seawater. The corrosion potential varies according to the reactivity of the specific metal. For example, zinc displays a lower (more negative) corrosion potential than iron, positioning it below iron in the galvanic series.
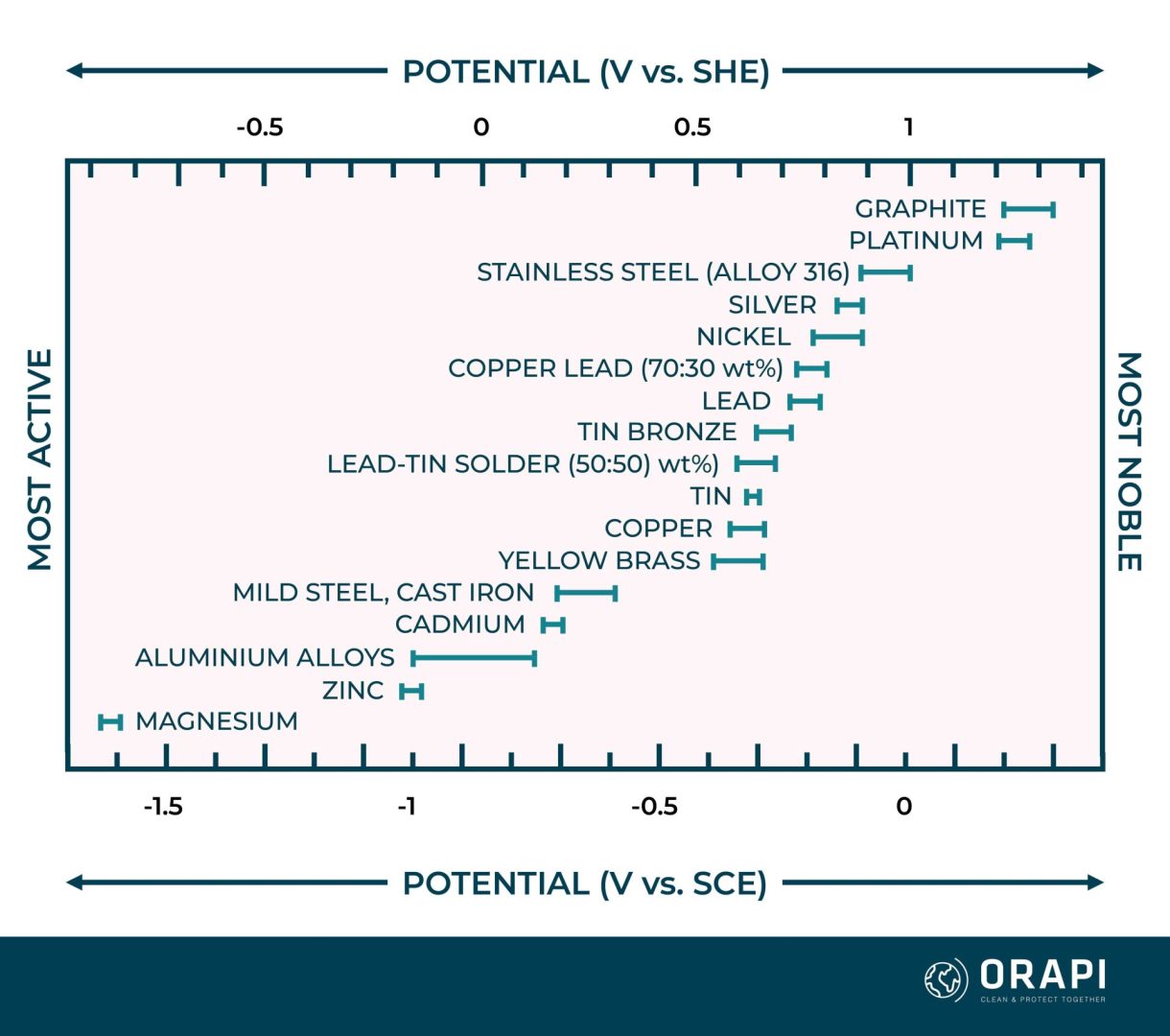
The corrosion potential is influenced by all the reactions taking place on the metal surface. Consequently, it depends on several factors, such as the electrolyte’s composition and the dissolved oxygen concentration. In Figure 1, each metal’s corrosion potential is depicted as a range of values rather than a single value due to the influence of these factors. Moreover, due to these variations, different sources of the galvanic series may present differences in the sequence of metals.
Tables or graphs illustrating galvanic series may sometimes feature stainless steel and certain other alloys in two positions. One position, typically labelled as ‘passive,’ corresponds to the alloy’s behaviour under normal conditions. This aligns with the position of stainless steel, as shown in Table 1 and Figure 1. The second position, designated as ‘active,’ is lower in the galvanic series and applies when the passive protective film of the alloy breaks down, which can occur in pit and crevice corrosion scenarios.
Metal Corrosion Due to Contact
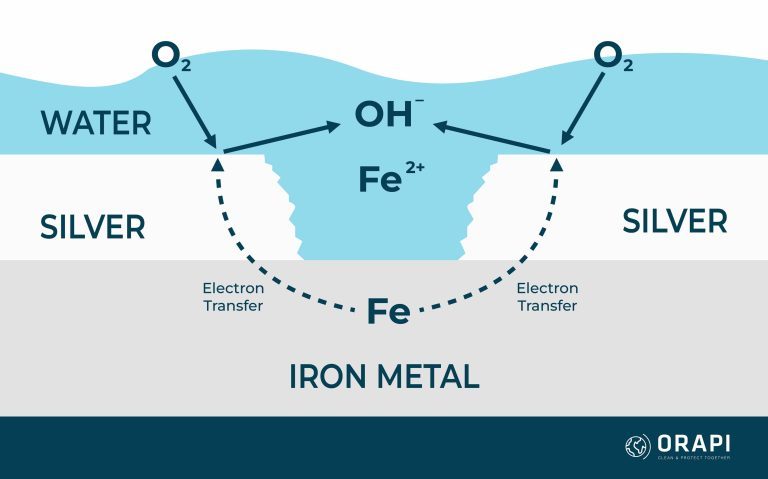
When two metals, such as silver and iron, come into contact within an electrolytic environment, they undergo a process equalising their electrochemical potentials. This equilibrium settles at a value between the corrosion potentials of the two metals, leading to alterations in their corrosion rates. In this scenario, the more reactive metal, iron, experiences accelerated corrosion compared to its standalone corrosion rate, while the nobler metal, silver, corrodes at a slower rate than it would independently. Simultaneously, the rates of cathodic reactions, such as the oxygen reaction, undergo counteractive changes; they speed up on the nobler metal and decelerate on the more active metal. This heightened corrosion of the more active metal, exemplified by iron in this instance, is commonly called galvanic corrosion. However, the galvanic series itself doesn’t quantify the extent of this corrosion rate alteration, although it is generally more pronounced when metals are positioned far apart in the galvanic series.
Figure 2 provides an illustrative example of these reactions, where a silver-plated layer has been compromised, revealing the underlying iron. In this case, corrosion is amplified by the relative surface areas of the two metals, with the exposed silver layer offering a substantial area for oxygen to engage in the corrosion process.
The Beneficial Side of Galvanic Corrosion
Galvanic corrosion can actually have a positive effect in some cases. While a more active metal will corrode more quickly due to this process, a more noble metal will corrode at a slower rate than it would on its own. Essentially, galvanic corrosion can be utilised to safeguard one metal from corrosion by giving up a more active metal. This more active metal is referred to as a sacrificial anode. For instance, metals such as zinc, aluminium, and magnesium, located beneath iron and steel in the galvanic series, have been employed as sacrificial anodes to protect steel in seawater. Sacrificial anodes were also used to protect a cast and wrought iron steam engine before its retrieval from a shipwreck in seawater. In addition, magnesium sacrificial anodes were placed in soil around an outdoor steel sculpture to safeguard the buried portions. Using a sacrificial anode to protect metal is just one example of a broader technique known as cathodic protection, which can also be achieved using a metal electrode and a power supply.
Active Plating

Galvanic corrosion occurs when one metal is plated onto another. The corrosion behaviour is determined by the relative activity of the two metals, specifically the plating metal and the underlying metal. An example of this can be seen in galvanised steel, where a layer of zinc is coated onto steel, resulting in the plating metal being more active.
Steel coated with zinc is protected in two ways: through a physical barrier and galvanic corrosion. If the zinc layer remains intact, it acts as a barrier against moisture, preventing the steel from corroding. Zinc, although more active than steel, is safeguarded by its corrosion products, typically zinc carbonate, which slows down its corrosion process outdoors. However, if the zinc layer is scratched or damaged in any way, the steel is exposed, and the zinc corrodes preferentially, thus protecting the steel through galvanic corrosion.
Galvanised steel offers protection against galvanic corrosion, thanks to the presence of zinc. However, when galvanised steel is used alongside other metals, zinc’s position below most other metals in the galvanic series can lead to rapid zinc corrosion. This can leave the steel vulnerable once most of the zinc has corroded away.
Steel can be protected using aluminium and cadmium, which are lower in the galvanic series. In addition, steel can be coated with alloys of aluminium and zinc. However, the use of cadmium to plate steel hardware on furniture has decreased due to its toxicity.
Noble Plating

Coating a more active metal with a more noble metal is a common practice to achieve the latter’s appearance. For instance, sculptures made of zinc can be copper-plated, brass stair railings can be chrome-plated, and copper trays can be silver-plated. Gold can also be plated onto silver or copper alloys to create gilded religious objects. Steel can be plated with various metals, such as copper alloys for brass bed frames, silver for kitchen utensils, chromium for automobile parts, tin for tin-plated cans, and nickel, before applying a layer of chromium on top. As long as the coating is continuous and free of porosity, only the more noble metal is exposed, acting as a barrier to protect the underlying metal from corrosion. If the more noble metal corrodes slowly, its protection can last for an extended period.
When the metal plating is flawed or damaged, the underlying metal can be exposed to corrosion. Galvanic corrosion, in this case, does not provide any protection, but instead, it accelerates the corrosion rate of the underlying metal. For example, copper-plated zinc sculptures may experience severe pitting around imperfections in the copper plating due to the galvanic corrosion of zinc, particularly in outdoor settings. Similarly, “tin cans” made of tin-plated steel can also suffer from galvanic corrosion of the underlying steel if the tin layer is damaged. The concentrated corrosion of the underlying metal can lead to the lifting of the plating layer if it occurs in a small area.
In some instances, unintentional plating can occur, leading to galvanic corrosion. For example, if a fountain with an aluminium sculpture contains copper ions from copper pipes, copper can deposit onto the aluminium due to electrochemical replacement plating. The areas where the copper has plated can then cause galvanic corrosion of the aluminium in the unplated areas, resulting in pitting. This same issue can also arise when copper plates onto zinc. It’s worth noting that even trace amounts of copper ions in water (as little as 0.1 ppm) can increase the corrosion rate of zinc.
Treatment of Galvanic Corrosion

Selecting Compatible Metals
When two dissimilar metals are in contact, selecting metals positioned as closely as possible to each other in the galvanic series is advisable. For instance, if the initial choice for the armatures inside a bronze sculpture is steel, it would be prudent to substitute it with stainless steel, a metal positioned higher in Table 1 of the galvanic series.
Insulating Materials
To prevent galvanic corrosion, one option is to use an insulator like Teflon or plastic to separate different metals. For example, a more noble metal sculpture could have a more active metal armature. However, if there are multiple contact areas, the failure of just one can cause the two metals to come into electrical contact. Additionally, if the insulator becomes porous as it ages, it can trap electrolytes like a sponge. This was seen in the Statue of Liberty, where asbestos separators soaked in shellac became porous over time. As part of the maintenance program, it’s important to regularly inspect the insulators between metals to prevent galvanic corrosion.
Plated Objects
When two metals are plated together, they cannot be separated. The object is inherently flawed if the plating places the more noble metal on top. This is because the problem of galvanic corrosion is built into the object itself. The only solution is to keep the object in an environment that won’t cause galvanic corrosion. Because the metals are permanently connected through an electrical connection, avoiding any connection through an electrolyte is crucial. The best practice is to store the object in a dry environment or protect it with regular waxing or other coatings.
Threatening the Structural Integrity
If galvanic corrosion is threatening the structural integrity of an object, the corrosion should be removed, or the corroded parts should be replaced. This can be a challenging task, especially for large sculptures. However, using a proper corrosion remover, you can restore the metal to its original form.
ORAPI RECOMMENDS:
OXIGO (f.k.a. OXIDEX) is a thixotropic one-step corrosion remover and surface renovator that prepares surfaces for refinishing and provides protection from further corrosion. OXIGO removes corrosion, degreases, etches and phosphatizes metal surfaces in one easy step.
Prevention of Galvanic Corrosion

Maintain a Dry Environment
In industrial settings, machinery, aeroplanes, equipment, etc., are often exposed to varying environmental conditions. At times, protecting them from highly humid conditions can be hard. However, by ensuring proper storage and sealing equipment from moisture sources, equipment can be protected from moisture to a certain extent.
Use Corrosion Inhibitor
Select a corrosion inhibitor compatible with your industrial equipment’s specific metals and alloys. It is essential to make sure that inhibitors demonstrate excellent performance in salt spray tests. In addition, inhibitors should be fast-drying as otherwise, the moisture from the product can be a source of corrosion. Lastly, the film formed should be easy to wipe off for cleaning and reapplication purposes.
ORAPI RECOMMENDS:
Anti-corrosion, water-repellent protective product that produces a semi-greasy film. (Available in red 804 and colourless 791).
Conclusion: Galvanic Corrosion
In conclusion, galvanic corrosion presents a significant challenge in materials and engineering, as it can lead to the degradation of metals when they come into contact with each other in the presence of an electrolyte. This article has delved into the causes and effects of galvanic corrosion, shedding light on the science behind it, including the galvanic series and corrosion potential. This article is a vital tool for assessing the corrosion susceptibility of various metals. While galvanic corrosion can lead to unsightly damage, it can also be harnessed for beneficial purposes, such as sacrificial anodes to protect more noble metals. However, when not appropriately managed, galvanic corrosion can threaten the structural integrity of objects. Prevention is the key, whether selecting compatible metals, using insulating materials, or employing corrosion inhibitors. Ultimately, understanding and effectively addressing galvanic corrosion is crucial in preserving the longevity and performance of various metal components and equipment.